With COVID-19 having an impact worldwide, International Filtration News is tracking stories relevant to the filtration industry. Filtration media for facemasks and respirators are at the forefront of the COVID-19 response effort, and meeting the demand for these types of filter media is proving to be a challenge. As manufacturers of filter media and others struggle to find ways to meet the need in the face of the growing COVID-19 pandemic, news stories are breaking quickly. Here we will post relevant news stories on an ongoing basis. Please check back for regular updates. If you have news that you feel should be added to this summary report, please email it to Matt Migliore at mmigliore@inda.media.
Most recent update: February 14, 2022
Nelson Labs announces verification program for facemasks and respirators
Nelson Labs, a leading global provider of laboratory testing and advisory services, has launched the Nelson Labs Mark, a verification program for product testing that authenticates test reports and provides data on product performance. The new offering is designed to empower consumers to make informed decisions when purchasing facemasks and respirators, as well as providing credible manufacturers with the means to differentiate their products from others on the market.
Amidst a rise in fraudulent products and the ongoing COVID-19 pandemic, Nelson Labs anticipates the program will become a valuable tool for hospitals, municipalities, and commercial buyers, along with private consumers. According to Nelson Labs, the Mark will begin with testing verification for masks and respirators but will expand to other products as the market requires.
“With varying regulatory requirements and amended qualifications during COVID-19, it is difficult for today’s consumers to discern legitimate products from those that have not been adequately tested,” said Jeffery R. Nelson, chairman, Nelson Labs. “The Nelson Labs Mark addresses this critical need for increased transparency by providing objective, verifiable performance data from our world-class laboratories.”
To further increase consumer confidence, the Nelson Labs Mark includes two-factor authentication for consumers to easily confirm the legitimacy of a product’s testing. The company will allow manufacturers participating in the Nelson Labs Mark program, whose products have undergone testing at Nelson Labs, to use the Nelson Labs Tested™ or Nelson Labs Verified™ mark on applicable product packaging and marketing, along with a corresponding web listing that includes detailed test reports outlining the performance of that product.
For more details on the program, visit nelsonlabs.com/get-verified.
Source: nelsonlabs.com
Texas Tech researchers author study using new standardized mask filtration testing to evaluate effectiveness of alternative facial coverings
Seshadri Ramkumar, a professor of chemical countermeasures and advanced materials in Texas Tech University’s Department of Environmental Toxicology, has co-authored a study utilizing standardized methods to evaluate common household fabrics as alternative materials for barrier face coverings. Olukayode James Ayodeji, a Ph.D. candidate in the Department of Environmental Toxicology, conducted the standardized tests measuring the filtration efficiency (FE) of different facial coverings.
Using mask filtration testing standards developed by the American Society for Testing and Materials (ASTM), Ramkumar and his team compared the performance of popular ready-made facial coverings, such as bandanas and neck gaiters, to alternative household materials including denim, cotton shirts, bedding and towels. Both categories of materials were measured against R95 masks, which offer the highest level of protection against viral particles.
While a material’s ability to filter out viral particles is paramount, breathability is an equally important consideration, Ramkumar said. Unfortunately, testing revealed an inverse relationship between filtration efficiency (FE) and breathability – for instance, while denim showed the highest FE, it also had the lowest breathability of all the household fabrics, rendering it unsuitable for facemask production. Ramkumar and his team took both parameters of FE and breathability into equal account when evaluating a material’s overall performance.
According to their study, the most effective ready-made facial coverings on the market are Velcro masks with carbon filters and surgical masks, while the least effective are fashion facemasks, single-layer face coverings, neck gaiters and bandanas.
The highest-performing household materials were thick cotton shirts and towels, indicating that multi-layered cotton facial coverings with proper fit are the most effective homemade mask alternative because they offer a measure of protection against viral particles without compromising breathability.
A combinatorial approach involving both a facial covering and a surgical mask with good fit would offer the wearer better protection against particle inhalation, Ramkumar said.
Ramkumar co-authored the paper, “Particle-Size-Dependent Filtration Efficiency, Breathability, and Flow Resistance of Face Coverings and Common Household Fabrics Used for Face Masks During the COVID-19 Pandemic,” alongside Texas Tech graduate students Olukayode James Ayodeji and Terrell A. Hillard, with the support of Texas Tech graduate student Mirza Khyum. The study was published in Volume 16 of the International Journal of Environmental Research earlier this month.
Source: tiehh.ttu.edu
Filtration devices could make dentist appointments safer during the COVID-19 pandemic
In a new study using 3D holographic imaging, University of Minnesota Twin Cities researchers tested the effectiveness of three filtration devices that can mitigate the spread of aerosols during ultrasonic scaling, a common dental cleaning procedure. The findings could increase health safety in dental offices during the COVID-19 pandemic.
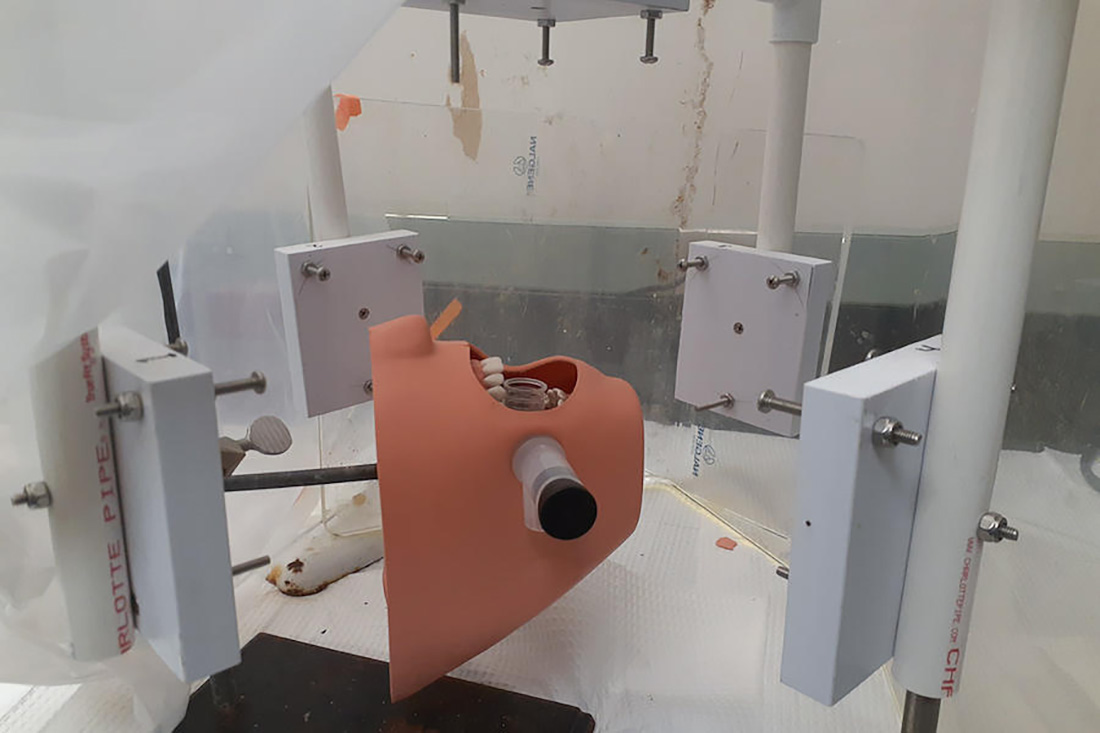
The researchers found that two of the devices—a high-volume evacuator and an extraoral local extractor—were very successful at reducing aerosol spread. This is one of the first studies to use advanced engineering imaging techniques to map the size, distribution, and mitigation of aerosols in dental offices.
The paper was published recently in the Journal of the American Dental Association, a peer-reviewed scientific publication from the world’s largest dental organization.
The University of Minnesota research team was led by College of Science and Engineering Professor David Pui and Associate Professor Jiarong Hong in the Department of Mechanical Engineering, with help from Professor Paul Olin, the Associate Dean of Clinical Affairs in the University’s School of Dentistry.
Read the full story: https://twin-cities.umn.edu/news-events/filtration-devices-could-make-dentist-appointments-safer-during-covid-19-pandemic
Source: twin-cities.umn.edu
Test results show ZENGuard coated facemasks provide 99.99% bacteria and virus filtration efficiency
Zen Graphene Solutions Ltd., a Canadian nanotechnology company focused on healthcare solutions, announced test results for bacterial filtration efficiency (BFE) and viral filtration efficiency (VFE) of its ZENGuard-enhanced surgical masks. The ZENGuard coated masks removed 98.9% more bacteria and 97.8% more virus particles compared to a typical ASTM level 3, 3-ply uncoated mask. With BFE and VFE levels over 99.99%.
- Testing was completed by GAP EnviroMicrobial Services Ltd., an ISO/IEC 17025:2005 compliant and Canadian Association for Laboratory Accreditation (CALA) certified testing facility
- A ZENGuard-enhanced mask and a leading ASTM level 3, 3-ply mask were exposed to S. aureus for BFE testing and MS2 Bacteriophage for VFE testing
- ZENGuard-enhanced mask removed 98.9% more bacteria and 97.8% more viruses in a head-to-head comparison with a leading ASTM level 3 mask
Source: zengraphene.com
US FDA updates enforcement policy for facemasks, respirators and related PPE to ensure availability
The U.S. Food and Drug Administration issued a revised guidance, Enforcement Policy for Face Masks, Barrier Face Coverings, Face Shields, Surgical Masks, and Respirators During the Coronavirus Disease (COVID-19) Public Health Emergency (Revised), to expand the availability of face masks, barrier face coverings and face shields for the general public, including health care personnel, as well as surgical masks and particulate filtering facepiece respirators (FFRs) (including N95 respirators) for health care personnel for the duration of the COVID-19 public health emergency.
This policy is intended to remain in effect only for the duration of the public health emergency related to COVID-19 declared by the Secretary of Health and Human Services (HHS) on January 31, 2020, effective January 27, 2020, including any renewals made by the HHS Secretary in accordance with section 319(a)(2) of the Public Health Service Act (PHS Act) (42 U.S.C. 247d(a)(2)).
Given this public health emergency, and as discussed in the Notice in the Federal Register of March 25, 2020, titled “Process for Making Available Guidance Documents Related to Coronavirus Disease 2019,” available at https://www.govinfo.gov/content/pkg/FR-2020-03-25/pdf/2020-06222.pdf, this guidance is being implemented without prior public comment because FDA has determined that prior public participation for this guidance is not feasible or appropriate (see section 701(h)(1)(C) of the Federal Food, Drug, and Cosmetic Act (FD&C Act) (21 U.S.C. 371(h)(1)(C)) and 21 CFR 10.115(g)(2)). This guidance document is being implemented immediately, but it remains subject to comment in accordance with the Agency’s good guidance practices.
Read the full guidance: https://www.fda.gov/media/136449/download
Source: https://www.fda.gov/
New research shows the interaction of water aerosol with nanofiber mesh facemask material
Facemasks made with nanofibers have drawn increasingly more attention because of their higher filtration efficiency, better comfort, and lower pressure drop. However, the interactions and consequences of the nanofibers and microwater droplets remain unclear.
New research published in the journal Physics of Fluids shows the evolution of fibers made of polymers with different contact angles, diameters, and mesh sizes under water aerosol exposure is systematically visualized. The images show that capillarity is very strong compared with the elasticity of the nanofiber. The nanofibers coalesce irreversibly during the droplet capture stage as well as the subsequent liquid evaporation stage. The fiber coalescence significantly reduces the effective fiber length for capturing aerosols. The nanofiber mesh that undergoes multiple droplet capture/evaporation cycles exhibits a fiber coalescing fraction of 40%–58%. The hydrophobic and orthogonally woven fibers can reduce the capillary forces and decrease the fiber coalescing fraction.
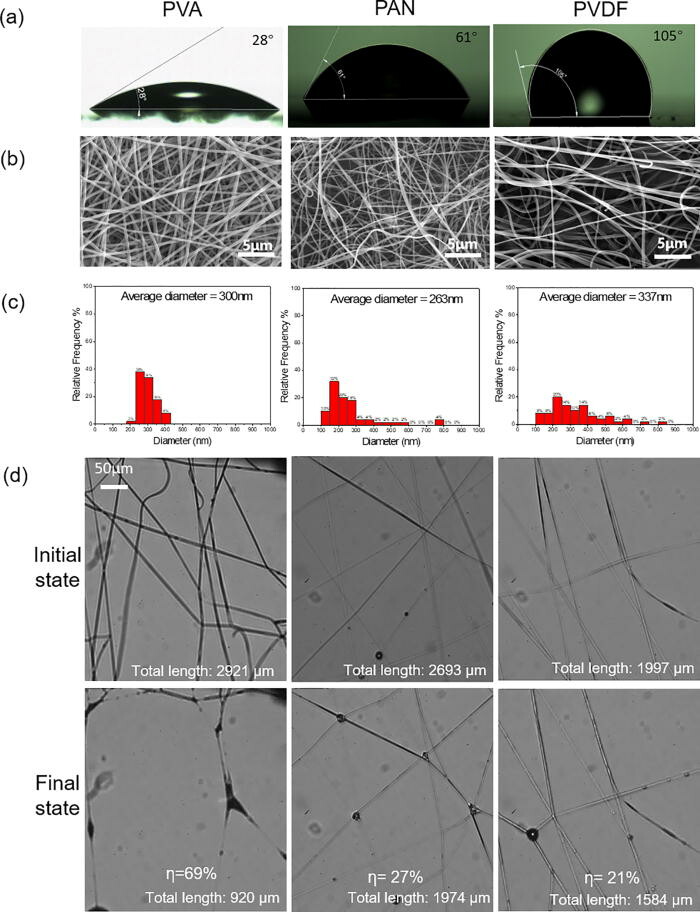
This finding is expected to assist the proper design, fabrication, and use of face masks with nanofibers. It also provides direct visual evidence on the necessity to replace face masks frequently, especially in cold environments.
Read the full story: https://aip.scitation.org/doi/full/10.1063/5.0061847
Source: https://aip.scitation.org/journal/phf
NCTO welcomes Senate passage of U.S. infrastructure bill, funding to bolster domestic PPE supply
The National Council of Textile Organizations (NCTO), which represents U.S. textile manufacturers from fiber through finished products, issued a statement welcoming Senate passage of a bipartisan infrastructure bill that will provide billions of dollars in new spending with the aim of revitalizing the nation’s roads, bridges and railways and help reconstitute a domestic supply chain for personal protective equipment (PPE).
“We commend the Senate for passing the bipartisan infrastructure bill, which will provide critical resources for our nation’s aging infrastructure and at the same time help incentivize the reshoring of personal protective equipment (PPE) production, an important priority of the U.S. textile industry,” said NCTO president and CEO Kim Glas.
NCTO worked with congressional allies to include a version of the Make PPE in America Act, legislation co-sponsored by Senator Rob Portman (R-OH) and Senator Gary Peters (D-MI), in the infrastructure legislative package. The bill ensures all PPE purchased by the Departments of Homeland Security, Health and Human Services and Veterans Affairs are Berry Amendment-compliant; guarantees long-term contracts (a minimum of two years) to U.S. manufacturers; and creates a tiered preference for PPE made in the Western Hemisphere by our free trade partners using U.S. components, after domestic manufacturing capacity has been maximized.
“We sincerely thank Senator Portman and Senator Peters for working to include their Make PPE in America Act in the infrastructure bill,” Glas said. “This bill will help onshore critical production of personal protective equipment (PPE) by guaranteeing long-term contracts for domestically produced PPE and ensuring that taxpayer dollars are utilized to bolster the federal purchase of American-made PPE.”
Source: ncto.org
Polymer nanothread filter captures 99.9% of coronavirus aerosols in experiment
A filter made from polymer nanothreads captured 99.9% of coronavirus aerosols in an experiment.
“Our work is the first study to use coronavirus aerosols for evaluating filtration efficiency of facemasks and air filters,” said corresponding author Yun Shen, a UC Riverside assistant professor of chemical and environmental engineering. “Previous studies have used surrogates of saline solution, polystyrene beads, and bacteriophages — a group of viruses that infect bacteria.”
The study, led by engineers at UC Riverside and The George Washington University, compared the effectiveness of surgical and cotton masks, a neck gaiter, and electrospun nanofiber membranes at removing coronavirus aerosols to prevent airborne transmission. The cotton mask and neck gaiter only removed about 45%-73% of the aerosols. The surgical mask did much better, removing 98% of coronavirus aerosols. But the nanofiber filter removed almost all of the coronavirus aerosols.
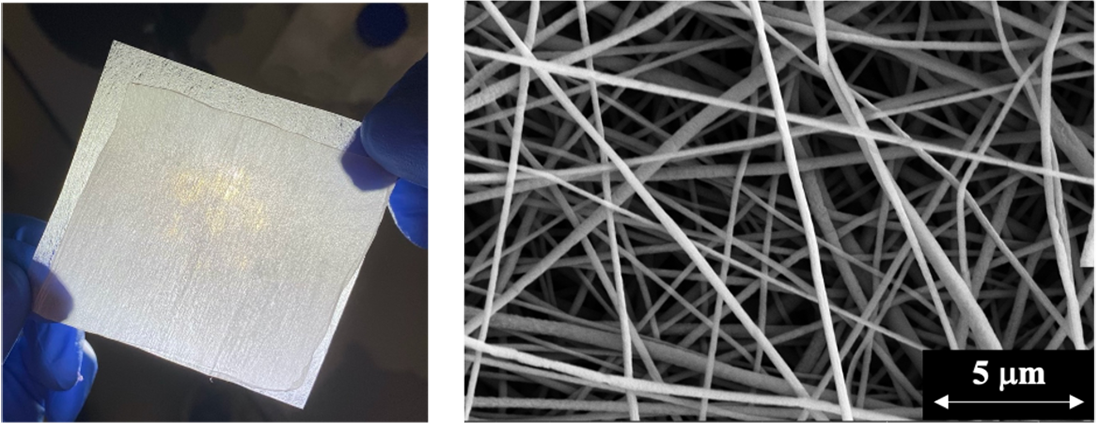
Shen and George Washington University colleague Danmeng Shuai produced a nanofiber filter by sending a high electrical voltage through a drop of liquid polyvinylidene fluoride to spin threads about 300 nanometers in diameter — about 167 times thinner than a human hair. The process created pores only a couple of micrometers in diameter on the nanofiber’s surfaces, which helped them capture 99.9% of coronavirus aerosols.
The production technique, known as electrospinning, is cost effective and could be used to mass produce nanofiber filters for personal protective equipment and air filtration systems. Electrospinning also leaves the nanofibers with an electrostatic charge that enhances their ability to capture aerosols, and their high porosity makes it easier to breathe wearing electrospun nanofiber filters.
Read the full story: https://news.ucr.edu/articles/2021/05/17/nanofiber-filter-captures-almost-100-coronavirus-aerosols
Source: ucr.edu
Meissner wins $13.4 million contract from BARDA to increase production critical to COVID-19 vaccine
Meissner Filtration Products announced the award of a $13.4 million contract from the Biomedical Advanced Research and development Authority (BARDA), part of the office of the Assistant Secretary for Preparedness and Response (ASPR) at the U.S. Department of Health and Human Services (DHHS), to facilitate expedited expansion of their production capacity for products critical to COVID-19 vaccine and therapeutics manufacturing.
“Meissner has experienced substantial demand for our products associated with federally funded vaccine and therapeutics manufacturing efforts,” stated Christopher Meissner, CEO of Meissner Filtration Products. “It has been incredibly fulfilling for our organization to be able to make a direct impact fighting this pandemic and this contract serves to amplify and accelerate those efforts.”
Meissner will be expanding its Camarillo, California, manufacturing campus by making a significant investment alongside the BARDA award to include additional cleanroom manufacturing space, implementing advanced inventory management systems and adding support areas to augment capacity.
“While we have seen increased demand on the basis of COVID-19 vaccines and therapeutics, our products are also a key piece in the development and manufacture of other lifesaving medicines so it’s critical that we able to support all of our customers through this period and in the future,” said Max Blomberg, Executive Director of Operations. “This expansion, which will provide a substantial capacity augmentation for our Camarillo campus, is just the latest in our continuing work to ensure we scale with our clients’ needs.”
This project has been funded in part with federal funds from the BARDA, ASPR, DHHS, under Contract No. 75A50121C00028.
Source: meissner.com
ASHRAE Epidemic Task Force releases ‘unequivocal statement’ on airborne transmission of SARS-CoV-2 in buildings
ASHRAE has released the following statement:
“Airborne transmission of SARS-CoV-2 is significant and should be controlled. Changes to building operations, including the operation of heating, ventilating, and air-conditioning systems, can reduce airborne exposures.”
It replaces the April 2020 statement that said airborne transmission was “sufficiently likely” that airborne precautions should be taken. At that time both, the World Health Organization (WHO) and the Centers for Diseases Control (CDC), contended that transmission of SARS-CoV2 was by droplet and fomite modes, not airborne. Subsequently, both have acknowledged the risk of airborne transmission indoors.
“This may seem like a small step, but we feel it is important to leave no doubt about our position, given the muted support for ventilation and filtration as important tools in the effort to stop the pandemic, from some organizations that should be leading more strongly,” said William P. Bahnfleth, Ph.D., P.E., ASHRAE Epidemic Task Force chair.
The ASHRAE Epidemic Task Force has been developing and disseminating guidance for the control of airborne transmission of SARS-CoV-2 since its formation in March 2020.
“ASHRAE volunteers have played a huge role in evaluating evidence and developing detailed guidance to improve indoor environmental quality,” said Bahnfleth. “The public, globally, is benefitting from the volunteer efforts of some of the most knowledgeable scientists and engineers in our field and this updated guidance is proof of it.”
To view the complete airborne transmission statement and other COVID-19 resources, visit ashrae.org/COVID-19. Questions specific to Epidemic Task Force guidance can be emailed to covid-19@ashrae.org
Source: ashrae.org
Researchers employ copper foam to filter microbes in facemasks and air filtration systems
During the COVID-19 pandemic, people have grown accustomed to wearing facemasks, but many coverings are fragile and not easily disinfected. According to research published in ACS Nano Letters, Georgetown University Department of Physics professor Kai Liu and his group of researchers have transformed copper nanowires into metal foams that could be used in facemasks and air filtration systems, which are more durable and have the potential to effectively filter out microbes.
“When a person with a respiratory infection, such as COVID-19, coughs or sneezes, they release small droplets and aerosolized particles into the air,” said graduate student James Malloy (C’22), first author of the study, “Minuscule particles can stay airborne for hours, so materials that can trap these tiny particles are ideal for use in face masks and air filters. The foams filter efficiently and can be easily decontaminated, reused repeatedly, and recycled.”
Though current filtration systems such as those in N95 masks are helpful in reducing the spread of COVID-19, the materials they are made with contain some drawbacks.
Fiberglass, carbon nanotubes and polypropylene fibers are not durable enough to undergo repeated decontamination procedures and some further rely on electrostatics that precludes washing, which leads to large amounts of waste from single-time use.
Liu’s team recently developed metallic foams with microscopic pores that are stronger and more resistant to deformation, solvents and high temperatures and pressures. As such, they wanted to test copper foams to see if they could effectively remove submicron-sized aerosols while also being durable enough to be decontaminated and reused.
Their most effective material was 2.5 millimeters thick and trapped 97% of deep submicron aerosolized salt particles, which are commonly used in facemask tests.
Read the full story: https://college.georgetown.edu/news-story/research-by-physics-professor-shows-copper-foam-could-be-highly-efficient-durable-filter-for-reusable-masks-and-air-cleaners/
Source: https://www.georgetown.edu/
Biden awards contracts for up to 22.2 million U.S. made facemasks
The National Council of Textile Organizations (NCTO) reported that the Biden Administration has awarded two contracts to Parkdale Mills and Ferrara Manufacturing Inc., as part of U.S. President Joe Biden’s pledge to procure millions of domestically made facemasks for community health centers, food pantries and soup kitchens across the country.
North Carolina headquartered Parkdale Mills, the nation’s largest cotton yarn spinner, has partnered with Ferrara Manufacturing, a tailored clothing company based in New York City’s garment center whose workforce is union represented by Workers United/SEIU, to manufacture over 17 million reusable masks.
The government said it could purchase up to a maximum of 22.2 million masks under the two contracts announced today. The masks will be Berry compliant, and thus 100% U.S.-made.
Ferrara Manufacturing and Parkdale Mills will contract with additional U.S. companies across the manufacturing supply chain, employing nearly 5,000 American workers as a result of these awards. Parkdale will be utilizing yarn from their facilities in NC, VA, and GA and Ferrara will deploy their cut and sew operations in New York City.
Source: ncto.org
Camfil to present webinar on managing virus threats with air filtration
On Thursday, April 8, Camfil will present a free webinar on how effective air filtration can help businesses, industrial facilities, healthcare facilities and public buildings mitigate virus infection spread. The virtual presentation will take place at 1:00pm EST.
The webinar will be led by Camfil’s Healthcare Segment manager, Kyle Petersen. With overall responsibility for providing the most compliant indoor air quality solutions to protect patients, visitors, and healthcare personnel in hospitals and other healthcare facilities, Mr. Petersen’s prior experience managing Camfil’s National Accounts Program has exposed him to a broad spectrum of air filtration in a variety of industry segments.
Key topics to be covered include:
- Air filter materials and configuration, and how they impact the ability of filters to mitigate virus risk
- Current scientific knowledge of the transfer dynamics of SARS-CoV-2 and other viruses
- The effectiveness of capture efficiencies at different MERV values
- The importance of MERV-A values in air filter choice
- Discussion of HEPA air filters and in-room air cleaners and air purifiers
Register for the webinar here.
Source: camfil.com
Ahlstrom-Munksjö, Virgis Filter S.P.A & Webasto partner to protect indoor environments from COVID-19
Ahlstrom-Munksjö has partnered with Italian filter producer Virgis S.P.A for the distribution of a HEPA filtration solution produced by Webasto, which is designed to make indoor environments safer against COVID-19 as well as other viral and bacterial organisms.
The HEPA devices, HFT300 and HFT600, were launched in Europe in 2020 to meet the demand for increased protection in vehicles and buildings. The main purpose of these filter devices is to protect the passengers and the operators in emergency vehicles, school buses and public transportation vehicles. The first units were primarily installed in ambulances in Germany and the U.S., as well as in public buses in the Netherlands and school buses in the U.S. Now the technology is being applied to offices, restaurants and museums.
The spread of COVID-19 and the consequent increase in the global demand for protective materials required many companies to rapidly adapt, leverage their expertise and innovate. Ahlstrom-Munksjö used its long-term experience and leadership in the manufacture of filters to develop a suitable media for HEPA H14, manufactured in a compact cartridge, which represents the core of the Webasto device.
The new HFT 300 and HFT 600 devices combine two main features to reduce viral loads in ambient air. They offer an extremely high virus removal efficiency thanks to HEPA H14 filters, and also generate very high air volume flow for the rapid and complete filtration of the air on an ongoing basis.
The HFT 300 and HFT 600 devices are specifically designed to filter the air, removing 99.995% of particulates corresponding to SARS/COVID-19 virus sizes (0.1 micrometers) and effectively reducing the risk of infection. Ultra-compact and light, these devices can be installed in less than 30 minutes.
The device complies with the International HEPA Filter standards WHO/CDC/ECDC, and is compliant with European Medical Device Directive CE 47/2007.
For more about HFT 300 and HFT 600 units from Webasto: https://www.webasto-comfort.com/int/content/air-filtration-system/
You can read more about HEPA filter from Ahlstrom-Munksjö: https://www.ahlstrom-munksjo.com/products/filter-media/air-filtration-for-industrial/high-efficiency-and-hvac/
Source: ahlstrom-munksjo.com
Nanocoating technology professes to stop SARS-CoV-2 in HVAC systems
A new nanocoating from Curran Biotech, based in Houston, Texas, professes to effectively and cheaply stop the spread and lingering impacts of the SARS-CoV-2 virus when applied to the filter fabrics in common HVAC (heating, ventilation and air conditioning) systems.
Curran’s Capture Coating permanently bonds to the porous materials within a filter, stopping viruses, bacteria and fungi dead, while not impacting airflow or breathability.
With the emergence of the worldwide pandemic, a new approach to protecting buildings and inside air from the transmission of contagions has been required, and the hydrophobic nanocoating was developed to combat the airborne nature of the coronavirus.
Standard air filters are rated using the MERV (Minimum Efficiency Reporting Value) system – with MERV8 typical for public buildings. With the Curran Biotech Capture Coating, these filters outperform much higher rated filters in terms of viral protection, without impacting airflow (static air pressure).
Curran Biotech treated filters operate normally with regard to dust and other dry particulates but reject aqueous/liquid-phase contaminants. Because the coronavirus in its most infectious state is surrounded by an aqueous liquid-phase environment, Capture Coating physically stops the virus at the surface of the air filter.
Capture Coating is being made available now from distributors and retailers across the globe. It is already being used in 11 states across the United States and it is to be expanded to 25 with the next few weeks.
Source: curranbiotech.com
Test results show Fiber Bond VE3 chemistry inactivates 99.9% of SARS-CoV-2 in HVAC systems
Fiber Bond announced the release of its VE3 Technology, an engineered chemistry that inactivates SARS-CoV-2, according to test results.
Lab testing concludes that Fiber Bond’s VE3 Technology inactivates 99.99% of the airborne SARS-CoV-2 virus within 15-minutes. The VE3 Technology is a value-added chemistry, developed in-house at Fiber Bond to help stop the spread of COVID-19 in any facility with an HVAC system.
The patent-pending VE3 Technology captures and inactivates the SARS-CoV-2 virus within the nonwoven media of Fiber Bond HVAC air filters. Fiber Bonds VE3 Technology was “evaluated for its ability to inactivate SARS-coronavirus type 2” by Microbac Laboratories, Inc., and “demonstrated a 99.99% inactivation of the virus challenged.”
In March 2020, shortly after the start of the COVID-19 pandemic, Fiber Bond’s team of engineers focused their expertise on the conceptualization and production of an HVAC-compatible filtration product with the ability to capture and eliminate the SARS-CoV-2 virus within an engineered binder system.
In-house research and development of VE3 Technology successfully and swiftly progressed at the Fiber Bond facilities in Michigan City, IN. And, by late 2020, Microbac Laboratories, tested the VE3 Technology for capturing and inactivating the airborne SARS-CoV-2 virus.
“This technology development undertaken by our Fiber Bond technical team is a testament to our ongoing efforts on providing products that enhance air quality. The VE3 technology is focused on providing an additional safeguard against airborne viral particles, specifically COVID-19, and is a great complement to our product portfolio that already includes antimicrobial products. Cleaner air helps to maintain a safer environment for all of us,” said Greg Wilkerson, president & CEO of Fiber Bond and Blocksom & Company.
The VE3 Technology is designed to work in residential, commercial, and industrial HVAC systems to provide equivalent protection against SARS-CoV-2 in high-efficiency air filters. The VE3 Technology is added to the adhesive bonding agent used in the manufacturing of Fiber Bond’s nonwoven filtration media for HVAC applications. VE3 also includes Fiber Bond’s exclusive EPA-certified Spor-Ax antimicrobial agent for eliminating fungal bacteria growth on the filter media.
Source: fiberbond.net
Guidelines doc provides ventilation strategies for COVID-19
The National Occupational Research Agenda (NORA) Manufacturing Sector Council’s COVID-19 workgroup published a guideline document highlighting building ventilation best practices to protect against the spread of airborne pathogens. The document, titled “Ventilation Strategies to Reduce the Risk of COVID-19 in Manufacturing,” is the product of a collaboration between the National Association of Manufacturers and the Centers for Disease Control and is based on input from individuals in business, labor, academia and government. The COVID-19 workgroup is primarily led and operated by non-government members, in order to tailor practices to manufacturing-specific needs. Shortly after the pandemic began, the workgroup assembled to identify information that was not readily available and would be helpful to keep workers safe.
The information is a collection of data, implementation strategies and testimonials on the use of outdoor air, existing industrial ventilation, and heating, ventilation and air conditioning (HVAC) systems to reduce the spread of COVID-19 at manufacturing facilities. These steps can be an important element in a facility’s health and safety plan when implemented within the Hierarchy of Controls, which are control measures, moving from most effective to least effective.
The virus that causes COVID-19 spreads between people more readily indoors than outdoors. Ventilation is a key engineering control at the point of the generation of infectious agents, where the source is infected people.
View the ventilation guidelines here.
Source: nam.org
SGS introduces IC Mark for reusable facemasks in U.S. and Canada
As more and more people around the world rely on the use of face masks to prevent the spread of COVID-19, SGS launched the first Independently Checked Mark (IC Mark) for reusable fabric masks, a new way for consumers in the U.S. and Canada to know if these products conform to industry and regulatory requirements.
The new IC Mark tests and verifies products using key performance attributes from the recently published industry consensus standard, ASTM F3502 Standard Specification for Barrier Face Coverings.

The Mark gives manufacturers and retailers a greater ability to demonstrate their focus on quality and performance, to create and supply products that meet or exceed the minimum legal requirements. In addition, its widespread use will provide consumers across North America with at-a-glance assurance that products satisfy safety and performance claims, and meet regulatory standards.
“Consumers need reassurance from manufacturers and retailers that the reusable fabric masks they are using are performing as intended”, says Matthew McGarrity, Senior PPE Technical Manager for SGS North America. He adds, “They also need to know important criteria such as how many laundering cycles their masks can go through until they are no longer of use.”
SGS in North America, along with other industry experts, helped draft the ASTM F3502 Standard Specification for Barrier Face Coverings. Its criteria include minimum design, performance, and care/use instructions.
Working with leading experts in textiles, filtration and restricted substances, SGS drafted various domestic and mask/face covering guidelines. The IC Mark approval process tests for:
- Breathability
- Particle filtration efficiency
- Labeling
- Strap attachment strength
- Service life claims
- Restricted substances
- Flammability
Source: sgs.com
New ASTM standard establishes minimum requirements for barrier face coverings in the US
According to report by CNN, a new national mask standard outlines minimum fit, design, performance and testing requirements for facemasks and would require user instructions, package labeling and a permanent tag on the product. ASTM International — an international standards organization — spent seven months conducting expedited testing and review and published its guidance on Tuesday. Experts and industry leaders say the new “Standard Specification for Barrier Face Coverings” has the potential to transform the quality of masks available for personal protection in the American marketplace.
“The new specification for barrier face coverings addresses a key gap and will support consumer confidence when purchasing a face covering that’s labeled as meeting the ASTM standard,” ASTM International told CNN in an email.
In a related story, CNN reports several members of President Joe Biden’s former coronavirus advisory board are urging his administration to more widely recommend and mandate the use of N95 masks, citing a “pressing and urgent need for action” driven by the threat of new coronavirus variants.
In a memo to Biden’s top coronavirus advisers obtained by CNN, a dozen health and safety experts — including four members of Biden’s former advisory board — called on the U.S. Centers for Disease Control and Prevention and the Occupational Safety and Health Administration (OSHA) to “recommend and require the use of respiratory protection, such as N95 FFRs (filtering facepiece respirators), to protect all workers at high risk of exposure and infection.”
They also urged the CDC to adopt the first national consumer mask standard and urged the administration to “coordinate a national effort” to distribute National Institute for Occupational Safety and Health-certified respirators and ASTM barrier face coverings to workers in need and use the Defense Production Act to ramp up mask production.
Source: cnn.com
NOTE: INDA, the Association of the Nonwoven Fabrics Industry, will present a webinar explaining the new ASTM F3502-21 Standard Specification for Barrier Face Coverings on Tuesday, March 2, 11 am EST. See the following brief for more details.
INDA adds webinar program to discuss new ASTM facemask standard for the general public
INDA, the Association of the Nonwoven Fabrics Industry, has added a date to its upcoming webinar series, Tuesday, March 2, 11 am EST, to discuss the new ASTM F3502-21 Standard Specification for Barrier Face Coverings, which was introduced by ASTM on February 15. This standard establishes a set of test methods that evaluate the filter, fit and leakage performance of barrier face coverings, commonly referred to as “facemasks,” worn by the general public and not to be confused with respirators nor medical or surgical masks. The webinar will be presented by respiratory expert, Jeff Stull, Vice Chair of the ASTM Committee that wrote the standard, and Dave Rousse, INDA President. It will provide a detailed review of the new ASTM standard and the test methods it entails and the impact on the entire supply chain of facemask production.
The purpose of guidance for the general public to wear facemasks is to control the spread of viruses. Facemasks made to this new ASTM standard will also provide a degree of particulate filtration to reduce the amount of inhaled particulate matter.
“We approached NIOSH last year on developing a general public facemask standard that could use nonwoven materials beyond Meltblown that still deliver an effective level of filtration, as there was so much demand for the N95 respirators and masks once the Asian supply chain was cut off,” said Dave Rousse, INDA President. “We were delighted to get a positive response from Jon Szalajda, NIOSH Deputy Director, National Personal Protective Technology Laboratory, who is also the Chair of the ASTM Committee dealing with standards in this area.”
“This was a worthwhile project that we worked through the ASTM process in record time,” said Szalajda. “It should provide an important benefit in the fight against COVID-19 spread by reducing consumer confusion about what works and what does not and assisting manufacturers in making effective products.”
The goal of the standard is to assist consumers in making informed decisions about facemasks given the vast array of products currently for sale, including various patterns promoted for home-made manufacture using common textile materials. Prior to the ASTM standard, no standard test method existed which allowed comparisons among different products nor were there any minimum performance requirements. This new standard provides these performance requirements as well as a set of specifications, guidelines and expectations for facemask manufacturers and media suppliers.
The Mar. 2 webinar will be in addition to the INDA Webinar Series already scheduled for March 16, March 25 and April 6. For information on the full webinar series, visit www.inda.org/inda-webinars.
Source: inda.org
* International Filtration News is owned by INDA, Association of the Nonwoven Fabrics Industry (inda.org).
Freudenberg Level 3 surgical masks receive U.S. FDA 510(k) clearance
ASTM Level 3 surgical masks manufactured by Freudenberg Performance Materials recently received 510(k) clearance from the U.S. Food and Drug Administration (FDA). The surgical masks are intended for use by healthcare personnel to protect both the patient and themselves from transfer of microorganisms, body fluids and particulate material. The facemasks are intended for use in infection control practices to reduce the potential exposure to blood and body fluids.
For the U.S. market, Freudenberg Performance Materials is now providing FDA cleared surgical masks meeting the Level 3 standard of the American Society of Testing and Materials (ASTM). The ASTM Level 3 surgical mask is for use in conditions where there is a high risk of fluid and spray of aerosol transmission, such as operating procedures. The masks are a single-use, disposable device provided non-sterile.
Surgical masks by Freudenberg were tested for performance in five areas: fluid resistance, differential pressure, particulate efficiency, bacterial filtration efficiency and flammability. Upon completion of testing, the surgical masks consistently met ASTM Level 3 criteria in all five performance test areas. Bacterial and particle filtration efficiency test results were greater than 99 percent.
The Durham, NC site of Freudenberg Performance Materials began mask production in 2020 in response to the COVID-19 pandemic. Working closely with local partners, Freudenberg is also aiming to prevent future PPE supply shortages by establishing long-term face mask production for the US market. The site manufactures surgical masks and community masks and is pursuing NIOSH N95 respirator certification.
Source: freudenberg-pm.com
Report predicts North American virus filtration market will grow 11.86% per year through 2026
The North America virus filtration market is expected to grow by 11.86% annually in the forecast period and reach $2,554.1 million by 2026, owing to rapid growth of pharmaceutical and biotechnology industry, increasing investments in R&D, surging need for virus removal and clearance amid COVID-19 pandemic. according to a new report by ResearchAndMarkets.com.
The report is based on a holistic research of the entire North America virus filtration market and all its sub-segments through extensively detailed classifications. The report is based on studies of 2016-2019 and provides forecast from 2020 till 2026 with 2019 as the base year.
Specifically, potential risks associated with investing in North America virus filtration market are assayed quantitatively and qualitatively through a Risk Assessment System. According to the risk analysis and evaluation, Critical Success Factors (CSFs) are generated as a guidance to help investors & stockholders identify emerging opportunities, manage and minimize the risks, develop appropriate business models, and make wise strategies and decisions.
Source: researchandmarkets.com
US DHS investigating counterfeit N95 facemask operation
Federal authorities are investigating a massive counterfeit N95 mask operation in which fake 3M masks were sold in at least five states to hospitals, medical facilities and government agencies. The foreign-made knockoffs are becoming increasingly difficult to spot and could put health care workers at grave risk for the coronavirus.
These masks are giving first responders “a false sense of security,” said Steve Francis, assistant director for global trade investigations with the Homeland Security Department’s principal investigative arm. He added, “We’ve seen a lot of fraud and other illegal activity.”
Read the full story: https://apnews.com/article/government-investigation-n95-scam-1694ed85d6ef99cdb6662f67d823c271
Source: https://apnews.com/
CDC research shows tight-fit masks or double masking with cloth and surgical masks increases protection
Wearing a mask — any mask — reduces the risk of infection with the coronavirus, but wearing a more tightly fitted surgical mask, or layering a cloth mask atop a surgical mask, can vastly increase protections to the wearer and others, the Centers for Disease Control and Prevention reported on Wednesday, as reported by The New York Times.
New research by the agency shows that transmission of the virus can be reduced by up to 96.5 percent if both an infected individual and an uninfected individual wear tightly fitted surgical masks or a cloth-and-surgical-mask combination.
Read the full story: https://www.nytimes.com/2021/02/10/world/double-mask-protection-cdc.html
Source: nytimes.com
Oerlikon Nonwoven, Wolf PVG commission meltblown plant for FFP2 masks
Oerlikon Nonwoven has commissioned a double-beam meltblown plant with ecuTEC+ electro charging unit at Wolf PVG GmbH & Co. KG. With this plant, the East Westphalian company can now provide nonwovens for the production of surgical and FFP2 masks. In addition to this filter material, which is in great demand today, high-quality meltblown nonwovens can also be produced for medical and industrial filter applications. The plant has now been running for several weeks under stable production conditions with optimal nonwoven fabric quality of the highest standards.

With the beginning of the COVID-19 pandemic and the shortage of protective masks that ensued, Wolf PVG GmbH & Co. KG, a wholly owned subsidiary of the Melitta Group, switched part of its production capacities to nonwoven mask fabric production. As a highly specialized system supplier for everything to do with vacuum cleaners and industrial filter technology, the company from East Westphalia can fall back on its extensive know-how and many years of experience.
With the meltblown plant from Oerlikon Nonwoven, Wolf PVG is further expanding its production capacities. The plant, with its two beams and the ecuTEC+ electro charging unit, is optimally designed for the production of face mask material. The plant is also ideal for the production of other filtration nonwovens. “A decisive point for investing in a plant from Oerlikon Nonwoven was the flexibility of the plant in relation to the possible product portfolio and the competence of the manufacturer,” said Markus Seele, COO of Wolf PVG. Dr. Ingo Mählmann, senior vice president of Sales & Marketing for Oerlikon Nonwoven, said, “Thanks to the numerous setting options for the electrostatic charge provided by the ecuTEC+, the optimum loading status can be set depending on the filter application.”
Source: oerlikon.com
ASHRAE Epidemic Task Force updates HVAC guidance for SARS-CoV-2
As the performance of many HVAC systems in buildings are still being evaluated, the ASHRAE Epidemic Task Force has updated its reopening guidance for HVAC systems to help mitigate the transmission of SARS-CoV-2.
“The Building Readiness Guide includes additional information and clarifications to assist designers and commissioning providers in performing pre- or post-occupancy flush calculations to reduce the time and energy to clear spaces of contaminants between occupancy periods,” said Wade Conlan, ASHRAE Epidemic Task Force Building Readiness team lead. “New information includes the theory behind the use of equivalent outdoor air supply, method for calculating the performance of filters and air cleaners in series, and filter droplet nuclei efficiency that help evaluate the systems’ ability to flush the building.”
Major updates to the building readiness guidance include the following:
- Pre- OR Post- Flushing Strategy Methodology: The strategy has been updated to include the use of filter droplet nuclei efficiency, which is the overall efficiency of filter based on viable virus particle sizes in the air, to assist in determining the impact of the filter on the recirculated air on the equivalent outdoor air. This allows the filter efficiency as a function of particle sized, using on ASHRAE Standard 52.2 test results, to be estimated based on the expected size distribution of virus-containing particles in the air. This calculation is currently based on Influenza A data and will be updated as peer-reviewed research becomes available for the distribution of particle sizes that contain a viable SARS-CoV-2 virus. Additionally, a chart added to help determine the time to achieve 90%, 95%, or 99% contaminant reduction if the equivalent outdoor air changes per hour is known.
- Flushing Time Calculator: There is now a link to a view-only Google Sheet that can be downloaded for use, to help determine the available equivalent outdoor air changes and time to perform the flush. This sheet is based on a typical mixed air AHU with filters, cooling coil, with potential for in AHU air cleaner (UVC is noted in the example), and in-room air cleaning devices. Provided efficiencies of MERV rated filters are based on the performance of over 200 actual filters from MERV 4 through 16, but the tool also allows users to enter custom characteristics for specific filters.
- This sheet also calculates the filter droplet nuclei efficiency based on the cited research but allows a user to adjust the anticipated distribution of virus as desired. It also allows specification of the zone (room) air distribution effectiveness from ASHRAE Standard 62.1 to account for the impact of the HVAC system air delivery method on the degree of mixing. Default calculations assume perfect mixing. Finally, the tool allows for the target air changes to be adjusted if an owner wants to achieve a different percent removal in lieu of the recommended 95%.
- Heating Season Guidance: the guide now includes data to consider for heating of outdoor air and the potential impact on pre-heat coils in systems.
- Adjustments to Align with Core Recommendations: The Core Recommendations were released in the last month and this guidance document needed to be updated to ensure that the information provided aligned with the intent of those recommendations. This included minimum outdoor air supply and filter efficiency requirements and their role in an equivalent outdoor air supply-based risk mitigation strategy.
The guidance still addresses the tactical commissioning and systems analysis needed to develop a Building Readiness Plan, increased filtration, air cleaning strategies, domestic and plumbing water systems, and overall improvements to a systems ability to mitigate virus transmission.
To view the complete ASHRAE Building Readiness guide and other COVID-19 resources, visit ashrae.org/COVID-19.
Source: ashrae.org
Porex medical device filtration materials shown to protect against spread of bacteria and viruses
Porex is one of the first in industry to initiate VFE testing for its porous polymer-based materials; results show components protect against certain aerosolized viruses
To address the spread of healthcare-associated infections (HAIs) from aerosolized viral particles, Porex Corporation, a global leader in porous polymers, has pioneered the use of VFE testing methods for materials used in medical device design. Likely the first in the porous polymer material science industry to run the new analysis on filtration media, the components were able to consistently obtain a 99.9987% VFE score, showing that its materials effectively help to protect healthcare workers and patients from aerosol-based viruses potentially present in medical settings.
Porex initiated VFE testing for its materials after observing the needs of its customers, which frequently fielded concerns from patients and healthcare workers seeking information that the components used in various equipment procedures would not pose risks of a HAI contraction.
Nearly one in 31 hospital patients acquires a HAI—and these infections lead to an estimated 99,000 annual deaths in American hospitals alone, according to the Centers for Disease Control and Prevention (CDC). Healthcare workers face similar—if not greater—threats due to frequent exposure to viral pathogens.
“The pandemic has magnified what was already a pressing issue in healthcare settings around the world, which is that inadequate filtration materials put patients and professionals at risk of contracting dangerous viruses,” said Avi Robbins, vice president, global product development and R&D at Porex. “We took the step to lead the materials industry into VFE testing to validate efficacy, and we are thrilled to confirm that our filtration and venting components are trustworthy and reliable for blocking viral particle spread.”
Porex develops venting and filtration solutions by leveraging several core technology platforms such as sintered particles, bonded fiber, PTFE and Oxyphen track-etched membranes. Filtration and venting media from Porex are suitable for suction canisters, catheters, syringes and other medical equipment utilized in aerosol-generating medical procedures.
Source: porex.com
MBT, Polyguard announce licensing agreement on nano silver antimicrobial tech for facemasks and filter media
MeltBlown Technologies (MBT) of Sandersville, Georgia, has entered an exclusive license agreement with Polyguard Group, to integrate Polyguard’s antimicrobial technology with MBT’s meltblown facemask and filter media.
In 2020, MBT was able to convert its traditional industrial and construction product model into producing meltblown filtration materials for facemasks. MBT was determined to find ways to improve on existing facemask materials and began collaborating with Polyguard Group to create meltblown products with antimicrobial properties.
“Once we met with the team at Polyguard we could see immediately that these guys were innovators. They have created one of the most unique and important antimicrobial technologies the world has ever seen.” said MBT president, Derek Yurgaitis. “I was completely blown away by the nano silver technology, its unique mechanism of action and the potential to be game changing in the fight against infections. Polyguard has already successfully inhibited Corona virus with a 99.15% reduction. We can now begin to imagine materials from HEPA filters to Facemasks that prevent microbes from replication on their surfaces.”
AFS FiltCon 2021 announces all-virtual format
Due to ongoing concerns surrounding COVID-19, as well as continued travel restrictions around the world, the American Filtration & Separation Society (AFS) has made the decision to shift AFS FiltCon 2021 to a fully virtual event.
The virtual edition of FiltCon 2021 will consist of two concurrent tracks, taking place over three days, April 19 – 21, 2021. The virtual setting will allow for conference attendees to participate in a total of 36 technical presentations, as all sessions will be available to conference registrants on-demand for two weeks after the live broadcast of presentations. Normally FiltCon provides four concurrent tracks with the ability for conference attendees to participate in a total of 24 technical presentations. By offering two concurrent tracks over three days, the virtual AFS FiltCon 2021 allows conference attendees to participate in a total of 36 technical presentations.
Source: afssociety.org
Mask-Alliance Bavaria certifies FFP2 facemask; production to begin at Zettl Group plant in Weng
Founded in May 2020, in response to the COVID-19 pandemic, the Mask-Alliance Bavaria established a complete value chain for the manufacture of facemasks in Germany. In its latest development, the Futurus facemask has been certified as an FFP2 protective mask. In December, production will start at alliance member Zettl Group’s plant in Weng, Bavaria.

Since the beginning of this initiative to produce mouth-nose protective facemasks, the founding members of the Mask-Alliance Bavaria – PIA Automation (automation specialist from Amberg), Sandler Group (manufacturer of high-tech nonwovens from Schwarzenbach/Saale) and Zettl Group (leading supplier in the field of automotive interiors) – had been working to produce certified FFP2 masks in Bavaria.
On December 3rd, Jörg-Timm Kilisch, Managing Director of DEKRA Testing and Certification GmbH, officially presented the certificate at the Zettl Group company site in Weng, Landshut district.
FFP2 or FFP3 masks are particularly recommended as personal protective equipment for healthcare workers who are exposed to an increased risk of infection in their work. The quality of the filter medium, the mask design, the fit and the available filter area are decisive for the classification of the product. In joint development work and close cooperation with the new association member DEKRA, the certification process was successfully completed at the end of October.
The fully automated production lines in Weng will now produce up to 6 million Futurus masks per month. An expansion of the production capacity to up to 10 million masks per month is already being planned. The Mask-Alliance Bavaria is thus expanding its contribution to securing the supply of these important medical products.
Source: masken-verbund-bayern.de
WHO issues updated guidance on mask wearing for COVID-19
The World Health Organization (WHO) updated its guidance published on 5 June 2020 based on new scientific evidence relevant to the use of masks for reducing the spread of SARS-CoV-2, the virus that causes COVID-19, and practical considerations. It contains updated evidence and guidance on the following:
• mask management;
• SARS-CoV-2 transmission;
• masking in health facilities in areas with community,
cluster and sporadic transmission;
• mask use by the public in areas with community and
cluster transmission;
• alternatives to non-medical masks for the public;
• exhalation valves on respirators and non-medical masks;
• mask use during vigorous intensity physical activity;
• essential parameters to be considered when manufacturing non-medical masks (Annex).
Read the updated guidance: https://apps.who.int/iris/rest/bitstreams/1319378/retrieve
Source: who.int
Why ventilation is key to battling COVID-19
As the weather gets colder and people head indoors, the risk of catching COVID-19 is rising. The Wall Street Journal published a video explaining why air ventilation and filtration are one of the best defenses against the coronavirus this winter. The piece does an excellent job of highlighting why MERV-13 filtration is important to indoor air quality.
Watch the video: https://www.wsj.com/video/ventilation-is-key-to-battling-covid-heres-why/EC6274D1-B4F0-40DF-A8EC-F9BDA7C5D1A1.html
Source: wsj.com
FILTECH 2021 postponed
With the expectation that Germany will be restricting large events through the first quarter of 2021 due to ongoing complications from the COVID-19 pandemic, FILTECH 2021, which was scheduled to take place Feb. 23-25 in Cologne, has been postponed. New dates have not yet been announced.
Conference organizers are working to identify dates to reschedule the event.
Source: filtech.de
HanesBrands facemask receives US FDA authorization
HanesBrands announced that a proprietary surgical facemask developed in conjunction with North Carolina State University, the University of North Carolina at Chapel Hill and UNC Health has been authorized by the U.S. Food and Drug Administration for use by health care professionals as personal protective equipment.
The two-ply, single-use surgical mask features a unique fabric developed by NC State’s Nonwovens Institute combined with a fit design created in collaboration with UNC-Chapel Hill and NC State biomedical engineers and UNC Health infection prevention experts.

The Hanes mask uses a duckbill shape for better breathability, a wire nosepiece and foam insert to enhance a contoured fit, and placement of stretchable straps for a secure fit. UNC Health tested the masks to assure they meet FDA particulate filtration standards and OSHA respiratory protection program requirements.
The FDA issued its Emergency Use Authorization for surgical masks in response to concerns relating to the insufficient supply and availability of disposable single-use surgical masks that provide a physical barrier to fluids and respiratory droplets.
The use of unique spun-bond fabric developed by the Nonwovens Institute, the world’s first accredited academic program for the field of engineered fabrics, eliminates the need for a third filtration layer for cost efficiency and filtering effectiveness. The spun-bond fabric is composed of two different polymer materials to make a single fiber that has significant strength and bulk that is as effective in filtration as current materials on the market.
Source: hanes.com/corporate
New company positions HEPA/UVGI air cleaning technology as an effective tool against COVID-19
A New Hampshire company, Air Cleaners Inc., has designed and developed a patented air cleaner that it says can play a critical role as we try to adapt to life in the age of COVID-19.
The company—formed by airflow experts from the semiconductor cleanroom and data center industry—was created to address aerosol transmission of pathogens using the experience and insight of its founders based on their decades of work modeling airflows in mission-critical facilities.
The company’s new product–the Clean Air Curtain(TM)–combines HEPA filtration and Ultraviolet Germicidal Irradiation (UVGI) with an “air curtain” high velocity exhaust to create a separation in local indoor airspaces. Their approach of utilizing HEPA level filtering together with UVGI lighting in a small, portable, desktop package represents a state-of-the-art process for producing the cleanest air possible while providing many unique and significant advantages, according to the company.
The Clean Air Curtain is complementary to local HVAC systems, as many buildings are without whole-building HVAC systems, or have inadequate fresh air ventilation. In these cases, the best method of clearing the air of pathogens is to use portable filtration devices.
Source: aircleanersinc.com
Data suggests cotton batting can increase the filtration efficiency of cloth facemasks
Recent FDA chief Scott Gottlieb, Ph.D., argued that he’d “rather try to get everyone in masks” and “try to get them in high-quality masks because we know it’s going to slow down the transmission.”
Against this backdrop, a new study published in Risk Analysis, “Reinventing cloth masks in the face of pandemics,” by Stephen Salter, P.Eng., describes how Effective Fiber Mask Programs (EFMPs) can help communities find a balance between the economy and curbing community spread.
A separate study by Stadnytskyi, et al. estimates that one minute of loud speaking generates at least 1,000 virion-containing droplets that remain airborne for more than eight minutes. If everyone uses effective masks, the benefit is compounded because each person’s mask reduces the number of particles they transmit, and also the number of particles they inhale.
The new study in Risk Analysis suggests that the effectiveness of cloth masks can be improved by using a non-woven material such as cotton batting. Increasing the surface area of fibers exposed to moving air improves filtering efficiency because the smaller particles are absorbed onto the fibers. In May and June of 2020, 17 handmade cotton batting masks underwent 35 tests using commercial quantitative fit testing equipment to determine their filtering effectiveness. The results showed average filtering effectiveness of 76 to 90 percent against aerosol particles.
Read the full story: https://www.sra.org/2020/10/23/new-data-on-increasing-cloth-mask-effectiveness/
Source: https://www.sra.org/
PureAir Filtration, Nobel Biomaterials partner to develop antimicrobial fiber for filtration
PureAir Filtration, a company specializing in removing corrosive gases, toxic vapors and odors, announced it has launched a strategic partnership with Noble Biomaterials, a leader in antimicrobial and conductivity solutions for soft-surface applications. The companies have developed an antimicrobial fiber called FiberShield that can be used as an added fabric layer in particulate filters to help fight microbes amid the COVID-19 pandemic.
FiberShield is made of a proprietary blend of nonwoven nanofibers that are impregnated with antimicrobial Ionic+™ silver technology. The antimicrobial fabric can be used in any particulate filter and is the only one on the market to offer such flexibility to filter manufacturers. FiberShield with Ionic+ technology has been tested and proven effective by independent testing laboratories to inactivate over 99% of specific pathogens.
PureAir also debuted a second product in its antimicrobial line called Microbe-sorb, an adsorbent media that utilizes a proprietary blend of compounds to activate, enhance and deliver the strong antimicrobial properties of permanganate, a material commonly used in medical practices since the early 1800s. Independent laboratory tests show Microbe-sorb inactivates over 99% of microbes on contact.
Source: pureairfiltration.com
Lidl to install hospital-grade air filters in all U.S. stores, aims to prevent spread of COVID-19
Following recently issued Centers for Disease Control (CDC) guidelines stating that the COVID-19 virus can be spread through the air, Lidl US announced it will install new air filtration systems rated MERV 13 or higher in all of its U.S. stores by the end of this year. Air filters rated MERV 13 or higher, which are typically found in hospitals, help filter out COVID-19, according to public health and industry leaders.
“Since the beginning of the pandemic, we have worked diligently to protect the health of everyone in our stores by meeting or exceeding CDC guidelines and this measure to create cleaner, healthier air is no different,” said Johannes Fieber, CEO of Lidl US. “Customers and team members in Lidl stores can breathe easier knowing we have an added layer of protection against COVID-19.”
Lidl US is among the first national grocery retailers to install hospital-grade air filtration systems across its entire store network. Previously, Lidl stores used advanced commercially rated MERV filtration systems. Epidemiologists and professional associations have recommended using high-efficiency air filters MERV 13 or higher wherever technically feasible to trap small airborne particles that can transmit the virus.
Source: lidl.com
Meat processing giant, Tönnies Group, leverages Camfil air cleaners as part of pandemic prevention scheme
Camfil has entered an agreement with Tönnies Group, Germany, for the implementation of an air filtration concept at a meat production facility at Tönnies headquarters. The aim is to set high hygiene standards for the sensitive food area and focus on improved indoor air quality at the facility.
In food production facilities, air is the invisible ingredient, and better air quality can help the facility achieve operational excellence and great quality product along with employee health. Often, the lack of standards in the meat production industry around ventilation and limitations in the design of the facility can lead to poor airflow along with insufficient positive pressure in the indoor environment. Also, to achieve the temperatures of around 6 C to 10 C required for meat processing, Tönnies uses convection coolers. They cool the circulating air and return it to the room for reuse. Scientific studies have shown, that airborne pathogens such as viruses can spread through this air recirculation. Germs packed in aerosols is 80 nanometers to 160 nanometers in size and can remain active in the air for up to three hours. These convection coolers can distribute the pathogens in the room and significantly increase the risk of infection for employees.

Due to the global pandemic, Coronavirus (COVID-19), Tönnies Group concluded that they want to protect their facility and employees from these harmful pathogens in the air. They aim to provide healthy, sustainable food with responsible production that meets high standards. The new multi-level hygiene solution consists of high-efficiency HEPA air cleaners, certified with EN1822:2019 from Camfil. These air cleaners (CC 6000 and CC 2000) with ProSafe HEPA H14 filters clean the indoor air from the circulating aerosols or viruses and provide clean filtered air to the cooling system for circulation. The systems were positioned at an elevated position so that the air cleaners can get a good flow of air through the production rooms and at the same time do not interfere with operations.
Initial measurements, which were carried out shortly after the installation, showed particle reduction by more than 50%.
Source: camfil.com
Nanotech coating for filters shows promise in capturing aerosolized virus particles
A physics professor from the University of Houston has developed a nanotech coating designed to allow air filters to capture airborne or aerosolized droplets of the virus that causes COVID-19.
The coating works by capturing liquids which encase the virus particles while still allowing air to flow through unimpeded. That allows ventilation systems to remove the virus during normal operation, without retrofitting or limiting the system’s ability to draw fresh air, said Seamus Curran, a physics professor known for his work commercializing nanotechnologies.

The coated filters are currently installed in one public building in New York City.
Balancing filtration with air flow is critical to indoor air quality, a key issue as colder weather in some parts of the country pushes more people indoors.
Curran, on the faculty of UH’s College of Natural Sciences & Mathematics, has worked with waterproof coatings, known as hydrophobic coatings, for almost a decade. The filter coating is a new water-based version designed to capture airborne virus particles, trapping them on the filter’s coating without limiting air flow.
He said the treated filters should be used in conjunction with other precautions, including masks and social distancing.
Read the full story at: https://www.uh.edu/nsm/physics/news-events/stories/2020/0929-filter-coating.php
Source: https://www.uh.edu/
Waterloo Filtration Institute to present Annual Conference with a focus on IAQ & COVID-19
The Waterloo Filtration Institute (WFI) will present its 2020 Annual Conference, December 15-16, 2020, 8:00 am -12:00 pm EST. The theme of the event will be “IAQ Health and Safety Solutions Associated with COVID-19”. It will address the critical roles of facemasks and air filtration during the current pandemic for public health and safety.
The virtual conference will feature the following four sessions:
1. Emerging Challenges and Responses
2. IAQ and the Built Environment
3. Facemask Technologies and Latest Developments
4. Facemask/Air Filter Test Methods and Standards
The event will feature 16 distinguished speakers from academia and the industry to share their latest developments and trends on IAQ health and safety solutions in response to the pandemic.
WFI is dedicated to supporting the growth of the global filtration industry and the advancement of filtration and separation processes for a clean, healthy, and sustainable world.
For full program details and to register, visit wficonference.org.
Freudenberg to add meltblown capacity in Europe
Freudenberg Performance Materials (Freudenberg) is expanding its production capacity for nonwoven media used exclusively to manufacture certified facemasks of the mouth-nose protection type pursuant to the EN14683 standard, as well as FFP1, 2 and 3 masks pursuant to the EN149 standard. The media are specially developed for and sold to the medical technology processing industry. To that end, Freudenberg is investing in the construction of a new state-of-the-art meltblown production line at the Kaiserslautern site. Commissioning of the new plant is slated for the first quarter of 2021.
“As a world-leading manufacturer of technical textiles and filtration media, Freudenberg Performance Materials has deep expertise in materials for certified face masks. By expanding our production capacity in Kaiserslautern, we are making a contribution to mastering the challenges presented by COVID-19 and supplying certified type face masks as well as FFP1, FFP2 and FFP3 masks in Germany and Europe”, said the CEO of Freudenberg Performance Materials, Dr. Frank Heislitz.
With the new line, Freudenberg is significantly expanding production capacity for meltblown nonwovens in Kaiserslautern. The world-leading technical textiles manufacturer produces these materials from extremely fine meltspun polypropylene microfibers that are several times thinner than a human hair. Masks made of these nonwovens can filter very small particles such as viruses thanks to the superfine structure of the material and its electrostatic charge.
Source: freudenberg.com
SWM increases production of air filtration media, as COVID-19 drives demand
In response to customer demand that has more than doubled over the past year, SWM International is ramping up production of its Alphastar Electrostatic Media product at its Wilson, North Carolina manufacturing facility. Alphastar Electrostatic Media is a primary component in HVAC air filtration products, including MERV 13-rated air filters.
“We began to see demand rising in April due to the COVID-19 pandemic, and quickly developed ongoing plans to support our customers. Expanding our output was critical, as we are an essential supplier to many of the largest air filtration companies in the U.S. SWM is determined to do everything we can to help our customers deliver the air filters and medical respirators that are vital tools in stopping infection spread and improving indoor air quality,” said Bart Sistrunk, Commercial Director of Filtration for SWM.
The company is expanding the workforce at the facility to support the higher production level and is continuing to evaluate further actions to increase output. This follows efficiency projects earlier in 2020 to increase capacity on the existing production lines. SWM anticipates ongoing demand for Alphastar media as commercial and residential customers respond to recommendations from the Centers for Disease Control (CDC) and ASHRAE (American Society of Heating, Refrigeration and Air-Conditioning Engineers) to upgrade building filtration to a minimum of MERV 13, which requires more frequent changes compared to lower level filters such as MERV 8.
The proprietary fiber blend in SWM’s Alphastar media is carded and needled into a fully homogenous material, making it a highly effective nonwoven for a variety of air filtration applications, including emergency respirators, N95 masks, CPAP (continuous positive airway pressure) machines, and ventilators.
Source: swmintl.com
Freudenberg facemask receives EN 14683 certification
Freudenberg Filtration Technologies announced its German-made facemask design has been certified as a TYPE II medical facemask according to EN 14683.
Freudenberg Filtration Technologies completed all the necessary certifications within a few weeks.
“At the heart of our medical face masks is a soft three-layer polypropylene filter medium that is characterized by its superior breathability,” said Dr Thomas Caesar, Director Global Filter Engineering Industrial Filtration at Freudenberg Filtration Technologies.
The three-layer design reduces the release of aerosols contaminated with viruses and bacteria. Freudenberg Filtration Technologies says that external laboratory tests confirm a filtration efficiency of more than 98% in aerosol separation.
The independent dermatological institute Dermatest has rated the product’s skin compatibility as “excellent,” according to Freudenberg. Elastic ear loops and a nose clip allow Freudenberg Filtration Technologies’ mouth-nose protection to be individually adjusted and ensure a comfortable fit.
Freudenberg is only supplying the certified facemasks to companies that order 7,500 units or more.
Source: freudenberg-filter.com
NWI’s spunbond high-efficiency filter media wins RISE Innovation Award
More than 150 professionals in product development, materials science, and new technologies convened for the 10th conference edition of RISE—Research, Innovation & Science for Engineered Fabrics, held virtually, Sept. 29-Oct. 1. The event was co-organized by INDA, the Association of the Nonwoven Fabrics Industry, and The Nonwovens Institute, and North Carolina State University.
Among the program highlights was the presentation of the RISE Innovation Award, which was presented to The Nonwovens Institute at North Carolina State University for its Spunbond High-Efficiency Filter Media. The product is a completely new approach to creating filtration media with the right efficiency at low pressure drop at a throughput of 350 kg per meter per hour. The pressure drop with The Nonwovens Institute’s new filter is unmatched by any meltblown structure and doesn’t require electrostatic charging, which has been an obstacle to facemask manufacturing during the COVID-19 pandemic. The materials is also much stronger than traditional meltblown filter media, thus providing the potential for reuse after appropriate cleaning and disinfection.
NWI won the 2020 RISE Innovation Award for its new spunbond filtration media. Photo: INDA
NWI said it has produced 4.5 million meters of the material so far, enough to produce 100 million masks.
Other nominees for the award included FemTech at MAS Holdings Pvt. Ltd. for its leakproof absorbent nursing pads and Sustainable Solutions Incorporated for its BlueCON Nonwovens initiative that produces recycled resin from hospital waste.
Source: riseconf.net
Researchers develop graphene filtration technology to neutralize organic particles, including bacteria, mold spores & viruses
Technology developed by researchers at Ben-Gurion University of the Negev (BGU) in Israel in partnership with Rice University in Houston, Texas is being commercialized by LIGC Application Ltd. to develop and manufacture products for filtration systems, including those that filter COVID-19 airborne particles.
LIGC is a company at the forefront of laser-induced graphene (LIG) commercialization. Hubei Forbon Technology Co. Ltd. (300387.SZ) in Wuhan, China provided $3 million in funding.
LIGC Co-founder and Chief Executive Officer Yehuda Borenstein says, “In the absence of better filtration technology, the indoor spaces where we used to spend most of our ‘normal’ life—schools, stores and workplaces— due to COVID-19 present a real risk. This technology will provide cleaner and more breathable air with lower energy and maintenance costs and virtually silent sound levels.”
Active air filters made with LIG are designed to damage and destroy organic particles including bacteria, mold spores and viruses at the micron and sub-micron levels when passed through a microscopic network of porous graphene.
This cost-effective and scalable approach is produced using commercially available CO2 lasers to create a conductive graphene mesh. The graphene mesh heats, electrocutes and neutralizes organic particles and pathogens with revolutionary efficiency compared to active carbon filters, UV-C and fiber HEPA filters that are used widely in schools, offices, homes, ships, and other facilities. Aircraft are already equipped with HEPA filters that remove viruses and bacteria from the circulated cabin air, but at high energy and maintenance costs.
Source: ligcapp.com
Source: aabgu.org
Lydall to add fine fiber meltblown line in France with capacity for 600 M respirators and 2.2 B surgical masks
Lydall, Inc., a manufacturer of value-added engineered materials and specialty filtration solutions, today announced its investment in a new production line to create fine fiber meltblown filtration media for facemasks and high-efficiency air filtration systems. This new line will be installed at Lydall’s facility in Saint-Rivalain, France, in the second quarter of 2021, substantially increasing the company’s capacity to supply this critically needed material to the European market.
The investment solidifies Lydall’s position as one of the largest global suppliers of fine fiber meltblown filtration media, the crucial component of N95 respirators and the European equivalent, FFP2/FFP3, as well as surgical masks and MERV-, HEPA- and ULPA-grade air filters. Lydall will receive up to 30 percent of funding for the investment through the support of France’s Ministry of the Economy and Finance.
“COVID-19 has created unprecedented global demand for face masks, upgraded air filtration systems and other products that are essential to preventing the spread of the virus. In response, governments around the world are now focused on establishing secure, reliable and sustainable domestic supply chains so they can guarantee they have access to high-quality products that are pivotal to national security and public health,” said Sara A. Greenstein, President & CEO of Lydall. “Our global footprint and 100-year heritage in creating specialty filtration solutions position Lydall to serve as a local manufacturing partner to governments around the world, now more than ever. We take our role in creating products that protect people and places from viruses such as COVID-19 incredibly seriously, and we are grateful for the support of the French government, which will allow us to ramp up production even further.”
Once this additional line is operational, Lydall expects to produce enough fine fiber meltblown filtration media for 600 million FFP2/FFP3 respirators or 2.2 billion surgical masks per year. The company plans to hire additional staff to support the increase in production.
Source: lydall.com
Polygiene and Hakers receive major order of facemasks from Dallas Cowboys
Polygiene and Hakers are partnered to produce facemasks using different Polygiene technologies. The first order for these masks comes from the Dallas Cowboys, with an order value of SEK 2.4 million. The masks will not be sold but used by the Dallas Cowboys and other organizations involved with the team.
For the initial order of masks, Polygiene’s Biostatic stays fresh technology is used. Biostatic stays fresh treatment works by soaking the material in a solution with antibacterial and antiviral properties. It reduces the bacteria by over 99% and this effect lasts over the lifetime of the garment. It has also shown to inhibit viruses, such as Norovirus, SARS (a type of Coronavirus) and Bird-flu virus. A treated product will reduce the virus by >99% over time, compared to an untreated material, according to the company.
For coming orders, Polygiene and Hakers are discussing the use of Polygiene’s ViralOff technology for masks and other products. Polygiene ViralOff is a brand for a treatment of textiles and other products that reduces viruses by 99% in two hours, according to the company.
“This is a good example of how antibacterial and antiviral functions have moved from being a concern for medical settings to something that concerns everyone post-corona. The partnership with Hakers means that many everyday uses and everyday concerns whether things should be washed ‘just in case’ will not be a problem,” said Mats Georgson, chief marketing officer for Polygiene.
Source: polygiene.com
Source: haksport.com
Revolution Fibres publishes paper touting electrospun nanofiber vs. meltblown for facemask filtration
Filtration plays an important role in purifying and decontaminating two life necessities — water and air. As awareness of the related health issues has increased, the demand for protection from airborne pollution and disease has also increased. From this perspective, Revolution Fibres of Auckland, New Zealand has issued a new white paper explaining the unique and enhanced capabilities that electrospun nanofibers provide when used as an active layer in facemasks. When compared to common meltblown filters, Revolution Fibres makes the case that electrospun nanofibres provide better protection against air particles, bacteria, and viruses such COVID-19.
Read the white paper: https://www.revolutionfibres.com/wp-content/uploads/2020/08/MB-vs.-NF-White-Paper.pdf
Source: https://www.revolutionfibres.com/
Colorado University researchers use wastewater to track and intercept community spread of COVID-19
This fall, when students in residence halls use the bathroom, they will be passively participating in important scientific research to keep campus safe.
Researchers at CU Boulder are setting up an anonymous observational network to monitor the wastewater leaving residence halls on campus as part of an effort to detect and intercept community spread of COVID-19.

According to Cresten Mansfeldt, project lead and assistant professor of civil, environmental and architectural engineering at CU Boulder, between 40 and 80% of infected individuals shed the SARS-COV-2 virus that causes COVID-19. That means what we flush down the toilet can contain the virus.
“It’s not a diagnosis, but could identify whether or not there are infections in certain areas of the campus,” said Mansfeldt. “It complements the entire framework being deployed at the university.”
That framework also includes individual testing, contact tracing and enhanced ventilation on campus.
The non-invasive wastewater surveillance system will be comprised of 23 sewer sampling stations on campus, run and tested by Mansfeldt and a team of 18 students and microbiologists.
Read the full story: https://www.colorado.edu/today/2020/08/27/how-sampling-campus-wastewater-aims-keep-covid-19-check
Source: https://www.colorado.edu
Sandler inaugurates new line for production of nonwoven filter media for facemasks
In early April, Sandler, the nonwovens manufacturer from Schwarzenbach/Saale, Germany, announced the investment in a new line to increase production of nonwoven filter media for facemasks and thereby expand its contribution to the security of supply of these products. On August 26, Sandler formally inaugurated a new production line for nonwovens for facemasks, with Hubert Aiwanger, Bavarian Minister of Economic Affairs, on site to mark the occasion.
The new line enables the production of filter media for about 600 million mouth-nose-protection masks a year and had started production on schedule in mid-August. Among many investment initiatives announced in response to the bottlenecks caused by the pandemic, this line is the first of its kind to come on stream to establish German supply chains for medical protective masks, according to Sandler.

Sandler’s CEO Dr. Christian Heinrich Sandler welcomed about 50 guests, emphasizing that it was and continues to be a matter of course for the company to contribute to overcoming the pandemic. “The main reason for investing in a new production line was our goal to increase our production capacity for nonwovens for face masks as quickly as possible. On August 14th, as we promised our Federal Minister of Health Mr. Jens Spahn, we began putting the new line into operation. In September, we will commence 24/7 operation,” said Sandler.
In May, Sandler AG, PIA Automation (automation specialist from Amberg), and Zettl Group (leading supplier of products for automotive interiors from Weng) had established the “Mask-Alliance-Bavaria.” Through this initiative, the companies set up an entire value chain for the manufacture of facemasks in Bavaria. Management representatives of both partner companies were also guests at the inauguration of the new production line in Schwarzenbach.
Source: sandler.de
US CDC & HHS to track COVID-19 cases through National Wastewater Surveillance System
The U.S. Centers for Disease Control and Prevention (CDC) and the Department of Health and Human Services (HHS), in collaboration with agencies throughout the federal government, are initiating the National Wastewater Surveillance System (NWSS) in response to the COVID-19 pandemic. The data generated by NWSS aims to help public health officials better understand the extent of COVID-19 infections in communities.
CDC is currently developing a portal for state, tribal, local, and territorial health departments to submit wastewater testing data into a national database for use in summarizing and interpreting data for public health action. Participation in a national database will ensure data comparability across jurisdictions.
Data from wastewater testing is not meant to replace existing COVID-19 surveillance systems, but is meant to complement them by providing:
- An efficient pooled community sample.
- Data for communities where timely COVID-19 clinical testing is underutilized or unavailable.
- Data at the sub-county level.
Wastewater can be tested for RNA from SARS-CoV-2, the virus that causes COVID-19.
Read the full story: https://www.cdc.gov/coronavirus/2019-ncov/cases-updates/wastewater-surveillance.html
Source: cdc.gov
The role of high-efficiency filters and laminar air flow
At the time of this writing, 800,000 people around the world have died from COVID-19. There have been 22 million reported infections. There is growing evidence that small aerosols are a major transmission source. Social distancing, inefficient masks, and MERV 8 filters are not a viable defense when dealing with a sub-micron enemy. Without some new strategy, another million people are likely to die. A study by McIlvaine Company shows the cost will be in excess of $20 trillion.
Read the full story: https://www.filtnews.com/high-efficiency-filters-and-laminar-air-flow-systems-needed-in-the-battle-against-covid-19/
AG Industries increases respiratory filter production by 1,500% amid coronavirus outbreak
AG Industries, which manufactures respiratory filters used in ventilators, oxygen concentrators, and CPAP/BiPAP machines, has executed a 1,500% increase in production of its bacterial/viral filters used for the treatment of COVID-19 patients.
With the demand for respiratory therapies continuing to surge due to COVID-19, AG Industries said it is well positioned to continue its role as a critical supplier for the world’s top ventilator makers, including those named in the U.S. Defense Production Act.

By partnering with the most respected global companies in respiratory therapy, AG Industries was able to execute its record increase in production over the course of three months.
“Our ability to ramp up production with such speed—utilizing private capital investments exclusively—speaks to the entrepreneurial nature with which we run our company and the trust that our customers have in us to deliver on our commitments,” said John Smits, president of AG Industries. “Most importantly, we are making a very real impact on the fight against the coronavirus by ensuring clinicians have what they need to treat every patient. We have had the privilege of helping the most sophisticated respiratory companies in the world identify improvements in critical therapies needed on the front lines of healthcare.”
In addition to its increase in ventilator filter production, AG Industries has successfully ramped capacity more than 50% for critical components utilized in oxygen concentrators. As the treatment profile for COVID patients shifts towards less invasive therapies, the demand for oxygen concentrators continues to surge, and AG Industries is actively partnering with its OEM customers to help meet this demand.
Source: https://www.agindustries.com/
ANDRITZ launches D-TECH technology for production of respiratory facemasks
Following its launch of the D-TECH line for production of surgical/medical masks in April 2020, ANDRITZ is now presenting its new D-TECH line for respiratory masks, such as duckbill and flat fold respirators.
The new ANDRITZ D-TECH respiratory mask line can be customized to laminate different layers of fabric, ensuring highest quality and hygiene standards. The line comprises unwinding and guiding units for nonwoven webs, automatic splicing of all raw materials, cutting and positioning devices for the metal nose bar, an edge welding and cutting unit, a 90° rotation process, positioning and welding of the ear loop elastics, as well as quality control using the D-TECH Vision System. Machine dimensions can be customized according to customers’ plant requirements.

Customers benefit from a fully automated production line including complete ultrasonic technology, a facility to include printing systems, and an interface to the automatic packaging machine. Moreover, there are different packaging options available – products can be packaged in bags by an automatic flow pack machine or packed in cardboard boxes by an automatic cartoner.
The respiratory mask line ensures a higher level of protection for the wearer as well as his environment due to its particle retention capacity (<0.3 µm) and the higher-quality defined total leakage compared to conventional protective masks. Producers already operating an ANDRITZ D-TECH surgical mask line can upgrade it to produce respiratory masks with a special kit.
Source: andritz.com
Two-layer polyester mask design shows 91.8% efficiency in Viral Filtration Efficacy test
Ed Goodwin, the founder and product development manager of HipSaver Inc., has always obtained scientific validation for all of his medical textile products. As such, he engaged Nelson Laboratories, to test his company’s DermaSaver Sci-Tex Mask construction.
“We believed our product would be effective based on the known textile science, and now these results from Nelson Labs have proven that to be true,” says Goodwin. “Virus transmission experts agree that exposure duration and dose volume of virus particles are two key factors in overwhelming the immune system. This test result shows a dramatic reduction in the dose volume exposure.”

Viral Filtration Efficacy (VFE) test showed the DermaSaver Sci-Tex Mask material construction to be an average of 91.8% effective in preventing aerosolized virus particles from penetrating through the mask. The virus particles tested are five times smaller than COVID-19 virus particles, implying that the efficacy against preventing COVID-19 virus particles from penetration could be even greater.
The mask, which retails for less than $20, consists of two layers of water resistant (hydrophobic) microfiber polyester that repels airborne droplets. The brushed inside surfaces of the layers mesh together to create an additional barrier.
For additional information and to view the full test report, visit: dermasaver.com
Study shows commercial air filters reduce presence of airborne particles with coronavirus
Surges in COVID-19 cases have accelerated research to find ways to reduce the spread of viruses, especially as airborne transmission seems more likely. American Air Filter, Co. Inc., and its subsidiary Flanders Corp. tested the effectiveness of its commercial air filters against a type of coronavirus, the Porcine Epidemic Diarrhea (PED) virus. The tests, conducted at the company’s Clean AIR Center in Jeffersonville, Ind., confirm that its commercial air filters reduce the presence of this virus surrogate in airborne particles, including in sizes similar to airborne droplets produced when a person sneezes, coughs or speaks.
The study was adapted from University of Minnesota research that showed a correlation between particle filtration and virus removal. That study used a porcine virus half the size of a typical coronavirus and used airflows much slower than are typical for a commercial system. AAF Flanders conducted this new research using PED virus, a member of the coronavirus family that behaves similarly, when airborne, to the virus that causes COVID-19.
Virus particles marked with a fluorescent dye were mixed with other airborne particles and drawn through individual air filters in test ducts at an airspeed mimicking commercial HVAC system airspeed. Samples of airborne particles were then collected upstream and downstream of the filter. Nikki Goss, M.S., manager of Biological Safety and Research, found that AAF Flanders air filters reduce the presence of the PED virus at approximately the same rate as overall particle removal.
“We have always been leading the way forward when it comes to filtration, but we never expected our expertise could aid in fighting a pandemic,” said Nathanial Nance, VP of Global R&D. “We take pride in knowing our products could reduce the risk of virus transmission.”
*AAF Flanders makes no claims regarding the ability of its filters to remove airborne SARS-CoV-2. Test results should be viewed as directional and real-world performance is environment-dependent. Methodology was based on prior tests comparing standard ASHRAE 52.2 testing to predictive analysis based on virus carriers.
Source: aafintl.com
Why high-efficiency filters are needed to support COVID-19 response in public buildings
Camfil has released a new video explaining MERV-13 air filters, the importance of a MERV-A rating, and advice for publicly accessible facilities.
Camfil HVAC air filtration experts, Mark Davidson and Greg Herman explain on this YouTube Video why commercial buildings facility managers need to use high-efficiency commercial filtration in public buildings after COVID-19 pandemic.
Source: https://www.camfil.com/en-us
Donaldson donates $100,000 to COVID-19 relief effort
Donaldson Company, Inc. has donated $100,000 to Direct Relief in support of global COVID-19 medical response and treatment efforts. The Donaldson Foundation grant will help humanitarian organization Direct Relief provide equipment and resources to medical professionals on the front lines who protect the world’s most vulnerable people.
Donaldson Foundation President Nathalye Blok said, “Whether it is sourcing and procuring masks or helping hospitals keep vital medical equipment operating, Donaldson Company and our employees have found numerous ways to help our communities during this unprecedented crisis. Inspired by the medical professionals battling COVID-19, we looked to Direct Relief, an organization that provides vital medical support and supplies to these dedicated professionals helping those who need it most, during times of crisis.”
Since January, Direct Relief has delivered more than 4 million N95 and surgical masks, more than 2 million gloves and tens of thousands of protective suits and other items to help safeguard healthcare workers in more than 69 countries.
For information related to Direct Relief’s COVID-19 efforts, visit https://www.directrelief.org/emergency/coronavirus-outbreak/.
Source: https://www.donaldson.com/en-us/
Testing the limits of facemask filtration
As the COVID-19 pandemic began to spread worldwide in the early part of this year, one of the first things to become clear was the supply of personal protective equipment (PPE) – facemasks in particular – was not ready for the ensuing surge in demand. In many parts of the world impacted by the coronavirus, facemasks were not commonly worn, and N95 was not a concept with which people were generally familiar.
Much has changed since then, and while there are certain countries where the use of facemasks and the understanding of their capabilities is still very much in the learning-curve phase, the demand has gone from select workplace environments to a scenario where a large portion of the global population is seeking facemasks for everyday use. This has stressed the supply chain for a variety of reasons, but it has also put significant pressure on the organizations who are involved in testing and validating the performance of various types of facemasks – from N95 respirators (and their equivalents worldwide) to surgical facemasks and others.
Read the full article: https://www.filtnews.com/testing-the-limits-of-facemask-filtration/
University of Cambridge Institute for Manufacturing working on dual, shared ventilator technology
In response to the COVID-19 pandemic, volunteers from the Institute for Manufacturing (IfM), University of Cambridge, responded to a request from clinicians at Royal Papworth Hospital to develop a device that, if needed in an emergency, could be attached to a ventilator to enable two COVID patients to receive tailored respiratory support.
Due to the success of the Government’s Ventilator Challenge UK, Royal Papworth Hospital NHS Foundation Trust, along with other NHS hospitals, received enough ventilators to meet the surge in demand from COVID-19 patients and continue to have a healthy reserve stock of ventilators.
However the new device – which is still in testing and not yet approved for clinical use – illustrates that it is possible to split the air flow from one ventilator to mechanically support the breathing of two sedated patients with different lung capacity and changing breathing needs.
- The new device, which is being developed in collaboration with Cambridge Design Partnership, improves upon and addresses concerns about previous ‘ventilator splitter’ designs, allowing one ventilator to be used by two patients more safely.
- The system incorporates respiratory parts that are available in the UK supply chain and which can be easily changed and replaced.
- Full design and testing details will subsequently be released so that the system can be developed for use in other countries.
Read the full story: https://www.ifm.eng.cam.ac.uk/news/new-ventilator-sharing-device-for-covid-19-patients/
Source: https://www.ifm.eng.cam.ac.uk
WHO issues updated brief on modes of transmission for COVID-19
The World Health Organization published a document updating its scientific brief published on March, 29 2020, entitled “Modes of transmission of virus causing COVID-19: implications for infection prevention and control (IPC) precaution recommendations.” The updated includes new scientific evidence available on transmission of SARS-CoV-2, the virus that causes COVID-19.
The brief provides an overview of the modes of transmission of SARS-CoV-2, what is known about when infected people transmit the virus, and the implications for infection prevention and control precautions within and outside health facilities. This scientific brief is not a systematic review. Rather, it reflects the consolidation of rapid reviews of publications in peer-reviewed journals and of non-peer-reviewed manuscripts on pre-print servers, undertaken by WHO and partners. Preprint findings should be interpreted with caution in the absence of peer review, according to WHO.
The brief covers modes of transmission for SARS-CoV-2, including contact, droplet, airborne, fomite, fecal-oral, bloodborne, mother-to-child, and animal-to-human transmission.
Read the full WHO brief: https://www.who.int/news-room/commentaries/detail/transmission-of-sars-cov-2-implications-for-infection-prevention-precautions
Source: who.int
Modeling airflow and filtration reveals truths and misconceptions about virus spread
The interior of an airplane is perhaps one of the safest places to be when it comes to avoiding contamination from a virus like COVID-19.
This is one of the surprising findings arising from detailed studies of recent so-called “super spreader” events in combination with computational fluid dynamics (CFD) modeling.
Together, they highlight the need for a new approach to advanced filtration in confined spaces, says Paul Bemis, CEO of CoolSim and Air Cleaning, Inc., based in Concord, New Hampshire, the developer of CoolSim, a leading cleanroom and data center software.
“When people realize the power of modeling airflow, they’ll understand how simple and valuable it is in keeping everyone safe from COVID-19 or any other airborne illness,” he says. “We’ve been doing this forever for cleanrooms, to avoid even the smallest particulates, and for data centers to manage energy use, but now we can help model how to manage what could be a life-or-death situation – the reopening of common spaces such as offices, restaurants and public buildings.”
Read the full article: https://www.filtnews.com/modeling-airflow-and-filtration-reveals-truths-and-misconceptions-on-commonly-held-beliefs-about-virus-spread/
European producers set for 20-fold increase in nonwoven facemask output by November
EU production of facemasks, essential for tackling the coronavirus crisis, is set to increase 20-fold by November this year compared to pre-crisis times. This means that EU-based producers will be able to make the equivalent of 1.5 billion three-layer masks a month, according to figures released by EDANA, the global association serving the nonwovens and related industries.
Over the last three months, EDANA has been liaising with partner associations including MedTech Europe, ESF, and EURATEX to ensure sufficient supplies of essential public health equipment. EDANA convened a new sector group representing face mask converters, nonwoven suppliers, testing laboratories and equipment manufacturers to work together to develop an independent and self-sufficient supply chain for medical face masks and personal protective masks in the EU. The group will work to ensure adherence to applicable European Standards and to encourage responsible product stewardship throughout the life-cycle of face-masks from raw material sourcing to end-of-life solutions.
Source: edana.org
Ahlstrom-Munksjö partners with sportswear company to provide filter media for facemasks
Ahlstrom-Munksjö established a partnership with Spanish sports and life style accessories company BUFF to provide filter media for face masks.
The BUFF facemasks are now available in the United States and Canada following a launch in Europe in June. Each BUFF facemask includes five single-use filters in the original packaging. Replacement filters are sold separately in packs of 30 units.
The spread of COVID-19 and the consequent increase in the global demand for facemask components required many companies to rapidly adapt, leverage on their expertise and innovate. Thanks to Ahlstrom-Munksjö’s long-term experience in the manufacturing of filter media and protective medical fabrics, the company has developed a new material suitable for Type II facemask applications. Leveraging on its capabilities and adapting its converting and packaging processes, Ahlstrom-Munksjö has established a partnership with BUFF to supply its replaceable filter media which is included in BUFF’s new range of facemasks.
Tests conducted on these new single-use filters have shown a 98% bacterial filtration efficiency and meet EN14683:2019 Type I & II standards. The new BUFF Filter Mask is lightweight, ergonomic and easily adjustable. Its features make it ideal to be worn also during sport and outdoor activities, protecting the user yet allowing a high level of comfort.
Source: ahlstrom-munksjo.com
University of Houston researchers have designed a “catch and kill” heated air filter that can trap the virus responsible for COVID-19, killing it instantly.
The nickel foam air filter was designed by Dr. Zhifeng Ren, the director of the Texas Center of Superconductivity at UH. Ren collaborated with Monzer Hourani, the CEO of Medistar, a Houston-based medical real estate development firm, and other scientists to design the filter. The original concept behind the filter that could eliminate COVID-19 was created by Medistar founder Monzer Hourani. Their unique design was outlined in a paper published in Materials Today Physics.
Read the full story: https://www.chron.com/local/article/UH-researchers-create-heated-air-filter-to-catch-15399250.php
Source: chron.com
Eagle Filters starts production of FFP2 and FFP3 facemasks
Eagle Filters announced its first facemask respirators are now being rolling out from the company’s production facility located in Kotka, Finland, with deliveries to the healthcare industry in the region coming soon.

Eagle Filters´ respirator product range covers respirators that meet both FFP2 and FFP3 class efficiency, with and without a valve.
Eagle’s unique filter material enables highly purified air, while keeping breathing resisitance low. The respirators’ sensitive exhale valve reduces moisture and heat buildup inside the respirator for a more comfortable user experience, according to the company.
Source: eaglefilters.fi
Camfil launches CamCleaner CC500 portable air purifier, targeting COVID-19 applications
Camfil, an air filtration research and engineering group, has launched the CamCleaner CC500. With HEPA filters thoroughly tested to a minimum of 99.99% efficient, and a 5-star MERV 9A pre-filter, the CamCleaner CC500 can be used as a portable air purifier or as a ducted negative air system to protect patients, staff and visitors from airborne viruses, pathogens, and harmful particles. In the fight against the spread of COVID-19, it can be used in places such as patient/resident rooms, procedure rooms, isolation rooms, doctor and dentist offices and retail spaces.
Some of the features of the CamCleaner CC500 include:
- 99.99% true HEPA (high-efficiency particulate air) filter
- Camfil 30/30 Dual 9 pre-filter, to extend the life of the HEPA filter
- Easy access filter replacement
- 34″ tall x 12.5″ wide by 14″ deep to fit into most spaces
- Weighs just 50 lbs, including filters, for manageable transportation
- Silent roll caster wheels or available with a mounting kit for wall- or ceiling-mount
- Hospital-grade cord set plugs into any standard 120-volt three-prong electrical outlet
- Delivers up to 12 air changes per hour (ACH) in 2500ft3 as an in-room HEPA air purifier
- Bottom air intake with purified air exhausted through slotted vent cap or optional duct

CamCleaner CC500 air purifiers with HEPA filtration efficiency for COVID.
The air in COVID treatment areas should be kept separate from the air in other hospital areas so that infected air droplets don’t escape into uninfected areas. As air flows from higher pressure zones to lower pressure zones, negative pressurization is commonly used to control the spread of airborne diseases in hospitals. When the air pressure in an isolation area is “negative,” it’s lower than the air pressure of surrounding areas, which means that outside air can flow into the area, but not out. This helps prevent infections from spreading but introduces a need for high-efficiency filtration within the area because of the lack of airflow. The CamCleaner CC500 is designed for to provide clean air in these challenging environments and others where COVID, or air quality in general, is a concern.
Source: cleanair.camfil.us
Considering pore sizes of surgical vs. respirator facemasks
Porometer has published an application note outlining test results using its POROLUX 100 porometer technology to test the pore size of surgical facemasks and N95 respirator facemasks. The application note explains how the pore size distribution of surgical facemasks and respirators can be measured using the “pressure scan” method employed by the POROLUX 100. The measurements show clear differences between the surgical mask and the respirator.

and a respirator (bottom). Photo: Porometer
Key findings include:
- Both masks’ outside layers have considerably larger pores compared to their middle layers. The middle layer is hence often called the filtration layer. In both masks, the middle layer has a more narrow pore size distribution compared to the other layers.
- Measurements show the differences between the middle layers of the surgical mask and the respirator, in terms of diameter and flow, respectively. The middle layer of the respirator has smaller pores than the middle layer of the surgical mask. The mean flow pore size (50% of the flow is through pores larger than the mean flow pore size) is smaller for the respirator (10.7 μm) than for the surgical mask (12.8 μm).
- The measurements are highly reproducible, proving the value of the POROLUX 100 for quality control purposes.
Read the full application note: https://www.porometer.com/application-notes/
Source: porometer.com
Suominen’s new material for facemasks meets EN standard for filtration efficiency & pressure drop
Suominen has developed a nonwoven material for the manufacturing of facemask applications. The new nonwoven has passed European Standard EN 14683:2019 Type II requirements in terms of filtration efficiency and pressure drop.
“Our FIBRELLA Shield nonwoven has excellent filtration efficiency and pressure drop values meaning that the material provides protection while being comfortable and easy to breathe through. Measured with an applied method by VTT* results indicate that FIBRELLA Shield nonwoven’s filtration efficiency is higher than 99% reaching type II requirements but of course the material can also be used for lighter model Type I masks or uncertified masks,” said Category Manager, Johanna Sirén.
The standard EN14683:2019 for medical masks is for end products and the converter has to repeat the tests to confirm the standard compliancy for the end product. The end product needs to comply also with the regional regulations, if any.
Developed in cooperation with VTT, this new material is the latest addition to the FIBRELLA family. FIBRELLA Shield is already in production at Suominen’s Nakkila plant. Currently the plant is capable of producing material for approximately 15 million masks per month.
Source: suominen.fi/en/
ATI adds EN 13274 filter testing compliance for FFP1, FFP2 & FFP3 masks
Air Techniques International (ATI), a leader in the design and manufacture of specialized testing equipment for HEPA filters, media, filter cartridges, respirators, and protective masks, announced the addition of European Standard EN 13274-7:2019 for Paraffin Oil to its 100X Automated Filter Tester lineup. The 100X automated filter tester with full EN 13274-7:2019 compliance has already been delivered to leading filter manufacturers from ATI’s global headquarters in Owings Mills, MD, USA.
EN 13274-7:2019 supersedes the previous standard (EN 13274-7:2008) and acts as the test method to determine particle filter penetration for respiratory protective devices. EN 13274-7 standard is the test method called by EN 149 for the testing of filtering half masks to protect against particles (FFP1, FFP2 & FFP3 masks), which are in high demand during the current global Covid-19 pandemic.

EN 13274-7:2019 complements the existing set of global standards that the 100X meets. “Our customers expressed strong interest in test equipment that is fully compliant with the new standard. We quickly developed a version of the 100X that precisely meets the aerosol concentration and particle size distribution required by the standard, and we have ramped up production to ship within 6-8 weeks to meet strong customer demand,” said Gautam Patel, Global Product Manager.
Source: atitest.com
Video highlights capabilities of ultrasonic machinery for facemask assembly
Sonobond Ultrasonics’ facemask assembly video demonstrates how this critical personal protection item can be created in approximately one minute, without thread or glue, enabling manufacturers to increase production speeds and reduce production costs. Sonobond’s ultrasonic machinery uses high-frequency vibratory waves to produce sealed seams and edges. The technology enables reliable bonds to be quickly created without stitch holes, glue gaps, fraying or unraveling, and no consumables are required.
Most commonly used facemasks can be wholly or partially assembled using Sonobond’s SeamMaster Ultrasonic Sewing Machine or a combination of the SeamMaster and the company’s PlungeBonder Ultrasonic Spot-Weld Bonder.
Disposable medical gowns and shoe covers, lint-free wound dressings, pillow and mattress covers, and pouches for sterilizing and storing medical instruments are some of the typical medical products assembled with Sonobond machines.
Watch the video: https://www.sonobondultrasonics.com/welders-bonders/nonwovens-textiles
Source: sonobondultrasonics.com
Study shows layered system of commonly available fabrics outperforms N95 respirator in filtration efficiency
A new study by Northeastern University found that a facemask constructed using fabrics manufactured by AKAS Textiles, a Pennsylvania-based textile manufacturer, outperformed an N95 respirator in an aqueous media under positive pressure of 20 Kilo Pascal, simulating a sneeze/cough. The study tested more than 70 different common fabric combinations and masks, including the N95 respirator, for their ability to block the transmission of virus-like nanoparticles. The mask with the best filtration was made of layers of ProCool Performance Fabrics combined with Zorb 3D Stay Dry Dimple fabric. The combination of these fabrics tested 72% more effective than the N95 respirator.
The study was published in ACS Nano, a monthly, peer-reviewed scientific journal published by the American Chemical Society. The authors wrote, “Layered systems of commonly available fabric materials can be used by the public and healthcare providers in face masks to reduce the risk of inhaling viruses with protection that is about equivalent to or better than the filtration and adsorption offered by 5-layer N95 respirators. The masks were evaluated with steady-state, forced convection air flux with pulsed aerosols that simulate forceful respiration.”

The study was done at 14 Liters per minute air flow, which is more than twice the human ventilation, at rest.
ProCool Performance Fabrics are two-sided fabrics, which are inter-knitted to provide filtration. The special wicking fibers in it help in managing the moisture. Zorb 3D Stay Dry Dimple is made with surface layers of wicking Stay Dry fibers with an innermost core of dense cellulosic fibers, which work as the filter. Th three-dimensional engineered pattern provides great filtration and keeps the mouth area dry and fresh, according to AKAS Textiles.
Read the research report published in ACS Nano: http://www.stevelustig.com/CV/COVID-Nano-preprint.pdf
Source: akastex.com
Research shows facemask textiles directly inactivate SARS-CoV-2
Researchers from Freie Universität Berlin at the Institute for Animal and Environmental Hygiene and the Institut für Textiltechnik (ITA) of RWTH Aachen University are collaborating on the topic of alternative personal protection equipment. The testing was conducted in the context of the EIT Health Project ViruShield, supported by the European Union, with the objective to discover alternative filter materials for facemasks in light of tight supply and globally imbalanced supply chains for personal protective equipment.
The Swiss company Livinguard has developed a treatment for textile facial masks that can directly inactivate bacteria and viruses, according to the researchers. While researchers at the Institut für Textiltechnik (ITA) of RWTH Aachen University conducted experiments on the chemical and physical properties of various textiles for face masks, researchers at Freie Universität Berlin were able to demonstrate that these new textiles can reduce high amounts of SARS-CoV-2 virus particles by up to 99.9% within a few hours.
The principle underlying the Livinguard Technology is that the textile surface has a strong positive charge. When microbes come into contact with the technology, the microbial cell, which is negatively charged, is destroyed, leading to permanent destruction of the microorganism. Unlike alternative metal-based solutions, the novel technology has been found to be safe for both skin and lungs. Moreover, Livinguard Technology is very sustainable, allowing users to reuse the mask up to 200 times with no reduced impact on safety or efficacy.
Source: ita.rwth-aachen.de & livinguard.com
Facemask line capable of producing up to 1M disposable surgical masks per day with a lower-cost design
The W+D/BICMA hygiene group of Winkler+Dünnebier has developed a fully automatic, high-speed facemask converting line for the production of high-quality disposable face masks. This new converting line will be able to produce masks for surgical-grade applications. It is capable of producing 800 facemasks per minute, but with a lower cost mask ear loop design.

The new AUXILIUM FM has a speed of up to 150 m/min (492 ft/min) and is able to produce up to 1 million high quality face masks per day. Adding to the manufacturing throughput efficiency is a unique lower-cost mask design capability for an expensive component of the mask, which maximizes the capabilities of lowest cost per piece mass production. There are also different options available for mask stacking and automation, which make this mask machine best suited for Industry 4.0 manufacturing.
The new mask line produces and laminates three fabrics (spunbond, meltblown, thermo-bonded nonwovens and others). It utilizes W+D/BICMA’s technology in automatic unwinding and splicing for nonwoven webs, cutting and positioning devices for the metal nose bar, and heat and ultrasonic edge welding.
Source: bicma.com
Lydall adds fine fiber meltblown line for filtration media for N95, surgical and medical facemasks
Lydall, a manufacturer of value-added engineered materials and specialty filtration solutions, announced an investment in an additional fine fiber meltblown asset in response to the exponential increase in domestic and global demand of specialty filtration media for facemasks. This new production line will enable Lydall – one of the few American manufacturers capable of producing high-quality fine fiber meltblown filtration media for N95, surgical and medical face masks – to significantly increase its supply and help alleviate the shortage of meltblown materials, both in the U.S. and internationally.
“In the wake of COVID-19, the need for the filtration media that makes face masks effective has increased dramatically, so much so that it is now being called the ‘golden fleece,’” Sara A. Greenstein, President and CEO of Lydall, said. “As one of the only companies in North America and Europe with the technical expertise, supplier relationships and access to the right machines to produce this filtration media, we feel great responsibility to do everything within our power to increase our output, support domestic supply chains and contribute to the global fight against COVID-19. This investment is one example of Lydall’s commitment to do just that.”
The new asset will complement Lydall’s existing global meltblown capacity and is estimated to supply the filtration media for one billion face masks per year, almost a third of the 3.5 billion that the U.S. Department of Health and Human Services has projected as necessary to protect healthcare workers. Lydall expects commercial production to begin in its Rochester, New Hampshire facility in the fourth quarter of 2020 and plans to hire up to 15 additional employees to support the increase in production.
Source: lydall.com
For more on Lydall’s COVID-19 relief effort: https://lydall.com/covid-19-relief-effort
Recommended wearing time of protective masks – researchers make better predictions possible with simulations
How long can you wear a protection mask? When will you have to change a soaked mask? Researchers of the Fraunhofer ITWM tackle these question using mathematical models and computer simulations. It is important to take into account not only the type of mask and the filter material, but also the physical stress level and personal features of the wearer.
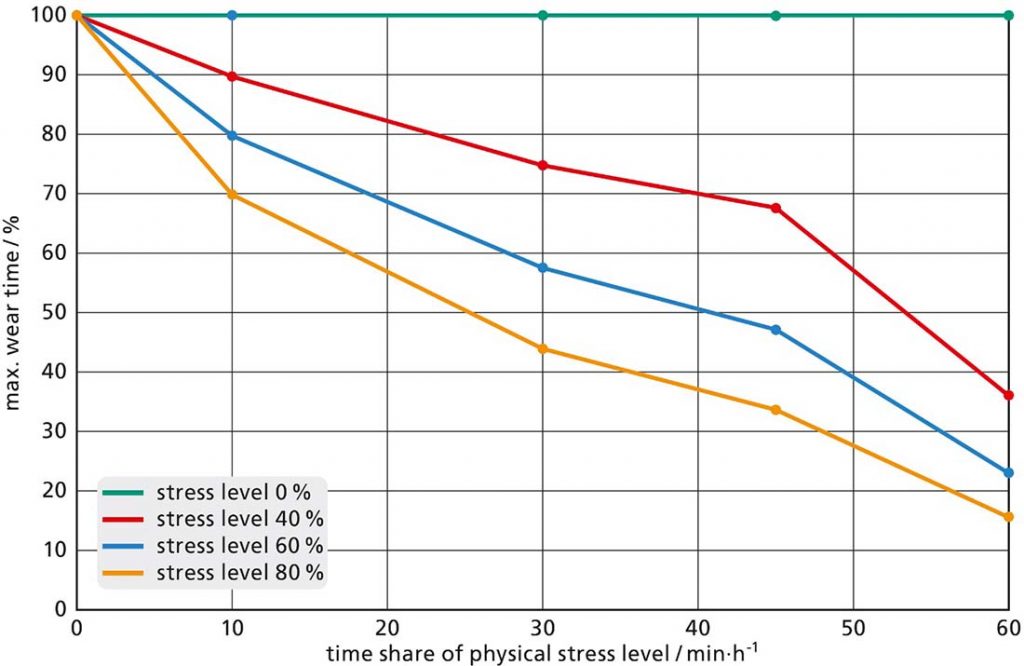
A reliable protection does not only depend on the filtration properties of the mask material. It is known that moisture penetration decreases the filtering efficiency and therefore, the safety is reduced. Humidity and body heat enhance the microbial contamination on the wearer’s side of the mask. The most critical state is reached when the mask is imbrued entirely (moisture breakthrough), since the moisture can serve as an “infection bridge” spanning across the depth of the filter material. This holds for highly efficient filter masks (e.g. FFP-2), but even more so for masks to protect others. Coughing and sneezing can lead to the detachment of potentially infectious droplets from the outer surface, which will then spread into the vicinity.
Read the full story: https://www.itwm.fraunhofer.de/en/press-publications/press-releases/2020_05_12_wearing_time_protection_masks_corona.html
Source: itwm.fraunhofer.de
Buildings in Boston are upgrading HVAC systems to prepare for COVID-19 reopening
Buildings in the Boston area are preparing for reopening by upgrading HVAC systems to prevent the spread of COVID-19, according to a report by Boston 25 News. The process is highlighting some challenges traditional HVAC systems face in preventing the spread of airborne viruses.
“When you recirculate that air, you have to be sure that air is going through a high-efficiency filter. Otherwise, any viral airborne particles will just be transmitted to another part of the building or an adjacent room,” Allen explained.
According to Allen, some property managers also plan to tinker with humidity levels. He said that is based on the concept that dry air in air-conditioned towers can more easily spread the virus.
“Relative humidity in a building is tricky because most building systems aren’t set up in a way that let you adjust relative humidity,” he said. “If you get it wrong, you can introduce conditions that lead to condensation and even mold growth.”
Read the full story: https://www.boston25news.com/news/health/buildings-upgrading-air-filtration-address-airborne-spread-covid-19/2MCDMDYR55A3XKPTNMVVHLCQ6U/
Source: boston25news.com
Denver-based screen company launches virus-filtering nanotechnology for filters and facemasks
Metro Screenworks, a Denver-based provider of retractable screen technology, introduced its VirusGuard virus-filtering material. VirusGuard can be utilized in several applications including creating masks, filters for masks, and constructing partitions and recently passed NH1 testing. VirusGuard is available in bulk rolls and ships internationally.
VirusGuard utilizes NanoScreen nanotechnology to restrict airborne, virus-carrying droplets, and is manufactured primarily to be made into masks and filters for masks. VirusGuard may help to increase the safety of personal masks as other safety materials may become scarce.

VirusGuard comes in two types, Type 9001 and 9002. Type 9001 has three layers and can be cut, sewn, and inserted into pre-made facemasks. Type 9002 consists of five layers and is ready to be cut and sewn into PPE masks. Both types can be hand-washed without degrading their functionality, though machine washing isn’t suggested. A Virus Guard mask can be worn 30 to 40 days with proper care. VirusGuard comes in white and black, though black requires extra manufacturing time.
VirusGuard is manufactured to be used for disposable and reusable safety masks. However, it lacks the resistance required for other applications like creating quarantine partitions. Allergy Guard, a related product, has the same potential virus-blocking capabilities as VirusGuard, but can be utilized for other applications, like constructing safety barriers and window screens, as well as creating filters.
Source: metroscreenworks.com
Considering MERV filter ratings & microbial filtration
Every filtration media is on a curve for filtration efficiency for particle sizes. Efficiency is dictated by operating velocity, and one filter can have a higher efficacy based on lower operating velocities. As we consider filtration in the age of COVID-19, an article of interest is a bit of a blast from the past from Air Media, a publication produced by our friends at the National Air Filtration Association.
Airborne microbes can be removed by filters at rates that depend on the filter performance curve and the mean diameters of the microbes. Each microbial species has a characteristic range of sizes that forms a lognormal distribution between the minimum and the maximum. The following diagram shows the fractional efficiency of different MERV values, providing a sense for what is possible from a filtration perspective.
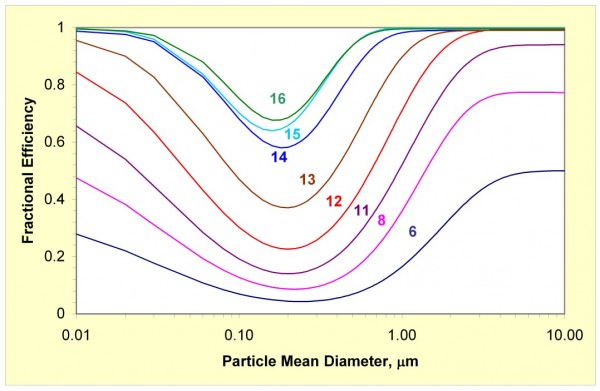
Read the full article: https://www.nafahq.org/merv-filter-models/
Source: https://www.nafahq.org/
Related article:
Filtration in the age of COVID-19
Ahlstrom-Munksjö launches Extia Protect range for facemask applications
Having successfully developed innovative solutions for facemask applications globally, Ahlstrom-Munksjö is now officially launching its Extia Protect product range, specifically designed for facemask applications.
The Extia Protect portfolio consists of a full range of high-performance fiber-based solutions for face masks, including filtration layers, cover stocks, lace media and reinforcement layers. Each component of the range has been designed to meet specific requirements of the different type of masks, including respiratory masks, surgical masks, but also civil masks. The facemask offering is produced on a global industrial platform consisting of plants in Europe, North and South America and Asia, giving the company the required capacity to meet the regional demand.
“I am very proud of the work accomplished by our team. In only a few months, we have developed a full offering for face mask applications and are now in a position to serve the growing demand for face mask materials globally by utilizing the available capacity we have in industrial platforms across the globe”, said Daniele Borlatto, EVP Filtration and Performance Solutions.
Source: ahlstrom-munksjo.com
For more on this product range: https://www.ahlstrom-munksjo.com/campaigns/extiaprotect
Berry announces capital investment in meltblown capacity in South America
Berry Global Group announced the capital investment in its global meltblown nonwoven fabric capacity for South America. This investment further strengthens the Company’s global reach and position as the leading nonwovens manufacturer. This line is Berry’s first meltblown asset, based on its Meltex technology, to be located in South America and continues to support the demand for health and wellness products.
With continued demand for face masks globally, Berry has been working closely with customers to help ensure production and supply. The investment will bring more than 400 metric tons of Meltex™ meltblown nonwoven material to the region, which will enable production of more than 500 million surgical-grade masks per year.
The new asset will be operational in the March 2021 quarter, will be placed at an existing Berry production facility in South America, and will focus on the production of materials for ASTM L2, L3, and N95 masks. The new line will be upgraded with Berry’s patented charging technology post installation.
Source: berryglobal.com
Xylem partners with UNICEF to help ensure sanitation and hygiene in at-risk countries as part of COVID-19 response
Xylem Watermark, the corporate citizenship program of global water technology company Xylem Inc., announced support for UNICEF’s COVID-19 relief efforts. The partnership will provide access to safe water and sanitation for vulnerable children and families. Xylem’s COVID-19 contribution will be directed to the most at-risk communities, providing critical supplies and supporting UNICEF’s community health programs and youth engagement initiatives. UNICEF’s COVID-19 water, sanitation and hygiene (WASH) response targets at-risk, low-capacity countries to secure WASH services and Infection Prevention and Control (IPC) in health facilities, schools, households and community settings.
Xylem Watermark’s support for UNICEF’s COVID-19 response is part of a broader commitment that aims to improve the health of low-income families through water, sanitation and hygiene education and infrastructure, around the world. The partnership builds on a decade of WASH educational programming, and scales the reach of the impact around the world to youth and communities most in need – through in schools, healthcare facilities and early childhood centers.
With UNICEF’s work in 190 countries, the partnership will contribute to achieving Xylem’s 2025 sustainability goals, including commitments to education and disaster-response, and to progress toward United Nations Sustainable Development Goal 6, which aims to “ensure availability and sustainable management of water and sanitation for all.”
For more information about Xylem’s COVID-19 support efforts: https://www.xylem.com/en-us/covid-19-response/global-coronavirus-actions/.
Source: xylem.com
Related article:
Filter media players step up in the fight against COVID-19, but questions about the long-term supply persist
Atlas Copco leverages 3D printing capabilities for facemask and face shield production
Atlas Copco Comptec, LLC, is supporting its local first responders by using its 3D printer to create facemasks and face shields.

Atlas Copco Comptec produces a range of standardized and customized large-scale compressors and turbo expanders. In addition to continuing to produce customer equipment, the facility has utilized its 3D printers to begin producing PPE, and in order to get the process operational quickly used readily available open-source 3D-printer files to produce masks and face shields. The 3D-printer produced mask is called a Montana Mask. 3D printers can produce six Montana masks with a single N-95 mask. The Montana mask can also accept HEPA filter material available at most hardware stores to create usable masks when N-95 media is not available.
Source: atlascopco.com
Camfil fields common questions about virus filtration
Camfil said it has been receiving questions daily from customers around the world about viruses in general and Coronavirus (COVID-19) in particular. As such, the company has gathered the most frequently asked questions about the Coronavirus (COVID-19) and filtration. Can the virus be caught in a filter within an air handling unit (AHU)? Do I need to change my filters after a virus outbreak? Which filter efficiencies are best at capturing viruses? Camfil’s vice president of R&D, Anders Sundvik, replies to these and many more questions.
Read the full FAQ: https://www.camfil.com/en-us/insights/life-science-and-healthcare/virus/frequently-asked-questions-about-viruses-and-filtration
Source: camfil.com
Market for virus filtration technology to reach $4.11 B by 2027
The global virus filtration market is forecast to reach U.S. $4.11 billion by 2027, according to a new report. Technological advancements in this filtration process and growing demand for single-use technologies are also supporting the expansion of the market.
With growing technological advancements, market players are coming up with the single-use designs in which the filter is gamma compatible and simple in terms of implementation in monoclonal antibody (mAb) processes and helps in conducting the research within the allocated fund by reducing the unnecessary allocation of budget to costly sanitization, according to the report. A number of market players are coming up with innovative products that eliminate the requirement for pre-flushing. Such advancements are a major driving factor supporting the expansion of the market.
The recent H1N1 Influenza outbreak, SARS outbreak, and anthrax attacks in 2001 have also resulted in increasing investment in research on these viruses by the governments of different nations, which is also fostering the demand for the process. The COVID-19 pandemic is driving additional traction in the market for virus filtration.
A free sample of the report is available at: https://www.reportsanddata.com/sample-enquiry-form/3032
Source: reportsanddata.com
Fitesa inks deal with Reicofil to expand its PPE capacity worldwide
Fitesa signed four new projects with technology supplier Reicofil. The package includes state-of-the-art equipment that will significantly increase capacity to serve the healthcare and hygiene markets, making nonwovens for medical gowns, drapes, surgical and N95 facemasks, diapers and sanitary products.
According to Fitesa, the exact locations of these projects will be disclosed in due time, but the intent is to strengthen the company’s global footprint without focus on any one region.
Source: fitesa.com
Freudenberg business units working together to produce and distribute facemasks for COVID-19 response
Freudenberg Filtration Technologies, Freudenberg Home and Cleaning Solutions and Freudenberg Performance Materials have joined forces to set up inhouse facemask production.
“Our objective was to fulfill our responsibility to our employees and society. We acted quickly, expanded our capacity and invested in production equipment for the manufacture of mouth-nose masks,” says Dr. Mohsen Sohi, CEO of the Freudenberg Group.
In addition to mask quality, the fair and needs-based distribution of the masks was an important factor for Freudenberg as it rolled out its mask production plan. As such, the company has limited sales on the Vileda online shop to a maximum order of two boxes per online customer will help ensure fair distribution and guarantee a wide dispersion that will benefit as many people as possible in the time of crisis.

Mask production is located in Germany and has been up and running since late April. Freudenberg Home and Cleaning Solutions is selling the masks under the name “Collectex”. The Freudenberg masks are made from a high quality, triple-layered filter medium. This filter medium is made from a high-tech nonwoven, which is also manufactured in Germany. The materials are processed into masks in newly acquired production lines launched in phases at Freudenberg Filtration Technologies – at first for the German market. Freudenberg is planning to expand capacity in the next few weeks to the point where roughly a million masks can be produced a day in four shifts around the clock seven days a week. The technology group will also continue to deliver media for the production of face masks to professional converters and existing customers.
Source: freudenberg.com
INDA launches portal highlighting nonwovens industry’s efforts in responding to COVID-19
INDA, the Association of the Nonwoven Fabrics Industry, launched a new portal on its website highlighting its member companies who are working to support the COVID-19 response worldwide. The portal, called “Allies Against COVID-19” is designed to connect suppliers with buyers and increases awareness of actions by nonwoven & engineered fabric producers in the fight against COVID-19.
The website details such actions from 71 INDA member companies that span the entire supply chain. The actions range from advancement in cutting edge viricidal and antimicrobial treatments to increased capacity and through-put to manufacture facemasks, gowns, disinfectant wipes, and community outreach. Companies are listed alphabetically and include contacts and website links.
“INDA is a resource connector in this effort to provide the necessary PPE materials and disinfectant wipes to combat the COVID-19 pandemic. We’re witnessing fast and strong contributions by the nonwoven & engineered fabrics community in this effort. INDA is proud to support our members by giving notice to their efforts to contribute to this noble cause,” said Dave Rousse, INDA President.
View the portal: https://www.inda.org/indamembers/covid.html
Source: inda.org
* International Fiber Journal is owned by INDA, Association of the Nonwoven Fabrics Industry
Chinese automakers introduce ‘virus-filtering’ air system
Chinese automakers are finding interesting ways to attract buyers after the major slump in car sales
caused by the Covid-19 pandemic. Chang’an Automobile — which operates joint ventures with the likes of Ford, Mazda, and Suzuki — shared on Wednesday that its “virus-filtering” air system for cars has reached mass production.
According to Chang’an, the high-efficiency filter can help keep passengers safer from pollutants and virus droplets in the air as they ride around town. The company received the CATARC CN95 certification back in April, meaning it can filter out 95% of particulate matter measuring 0.3 micrometers or larger. The company claims it can filter out particulate matter down to 0.1 micrometers. Most viruses, however, count their sizes in nanometers, which is a thousandth of the size of a micrometer.
Read the full story: https://www.abacusnews.com/china-tech-city/chinese-carmakers-introduce-air-filtration-systems-stop-viruses/article/3083324
Source: abacusnews.com
ASHRAE issues guidelines for reopening buildings during COVID-19 pandemic
The ASHRAE Epidemic Task Force has developed guidance on mitigating potential health risks during reopening of buildings closed during the COVID-19 pandemic.
ASHRAE’s recommendations for reopening buildings are outlined in the frequently asked questions section of its COVID-19 Resources webpage. Recommendations for building readiness and reopening include the following:
- Create a strategic plan prior to opening a building. The plan should include measures to make occupants feel safer, ensuring supply chain for critical items such as filters and communication plans for building support and safety measures for occupants.
- If the building opening takes place when Personal Protective Equipment (PPE) requirements are still in place, ASHRAE’s Occupancy Guides can be referenced to deal with functioning buildings during the epidemic.
- Review HVAC programming to provide flushing two hours before and post occupancies. This includes operating the exhaust fans as well as opening the outside air dampers. For buildings without the capacity to treat large quantities of outside air and when outside air conditions are moderate, open all windows for a minimum of two hours before reoccupation.
- Ensure that custodial scope includes proper cleaning procedures built from EPA and CDC guidance on approved products and methods:
- Disinfect high-touch areas of HVAC and other building service systems (e.g. on/off switches, thermostats)
- Disinfect the interior of refrigerated devices, e.g. refrigerators, where the virus can potentially survive for long periods of time.
- Run the system on minimum outside air when unoccupied.
- Garage exhaust, if any, should run two hours before occupancy.
Source: ashrae.org
The Nonwovens Institute announces COVID-19 webinar series
The Nonwovens Institute will be presenting a series of six webinars providing critical technical information and insight on the value and importance nonwovens technology for infection prevention.
The first webinar, “The Anatomy of N95 and Surgical Masks,” will take place May 20, 2020, 11:00am – 12:00pm EDT. Registration is free for NWI Members and $50.00 for nonmembers. Dr. Behnam Pourdeyhimi, the executive director of the The Nonwovens Institute, will examine the specific materials, construction, and performance of various facemasks in light of the current Covid-19 pandemic. He will discuss the key attributes of nonwovens technology and their effect on filtration efficiency and user protection.
Registration Link: https://reporter.ncsu.edu/link/courseview?courseID=NWI-WBR-01&deptName=NWI
Other webinar topics include:
- Webinar #2: Air and Aerosol Filtration Basics
- Webinar #3: Meltblown Fabrics and Process in PPE
- Webinar #4: Spunbond Fabrics and Process in PPE
- Webinar #5: Gowns
- Webinar #6 Wipes and Other Products for PPE.
Source: thenonwovensinstitute.com
Techmer PM develops technology to help meltblown fabrics maintain electrical charge
As demand surges for facemasks during the COVID-19 pandemic, Techmer PM, LLC, has developed a technology to improve the efficacy of the fabrics used in the production of such masks.
The technology, called the Charge Enhancer, is used during the production of meltblown nonwoven fabrics to help the resulting masks meet the filtration performance requirements set forth by ASTM F2100.
Techmer PM provides its Charge Enhancer in pellet form to producers of meltblown nonwovens, who then add it to polypropylene in a concentration of 4- 5%.
As the extruded nonwoven comes off the production line in roll form, the material is subjected to an electrical charge via an air plasma treatment, also known as “corona” charging (no relation to the coronavirus). The Charge Enhancer technology helps the meltblown fabric enhance and retain the electrical charge imparted by corona charging.
“Without the Charge Enhancer, the mask media would struggle to retain a filtration efficiency of ≥95%,” said Bhushan Deshpande, Techmer PM’s vice president of technology.
Techmer PM is also testing new technology designed to provide more permanent charge-enhancing effects compared to what is currently available. “This newer technology will be well suited for mask manufacturers looking to develop reusable masks,” according to Deshpande.
Source: techmerpm.com
Midwest Textiles, Hollingsworth & Vose partner to develop homemade facemask kit
Midwest Textiles and Hollingsworth & Vose (H&V) are collaborating on a new ready-to-sew face mask kit for the general public. The new collaboration between Midwest and H&V offers an improvement to the everyday consumer by adding a layer of Nanoweb FM to the mask.
Nanoweb FM is new filtration media made by H&V, designed for use in homemade face masks.
“H&V is one of the world’s leading producers of filtration media for face masks and many other filtration applications. By partnering with Midwest, and through the development of Nanoweb® FM media, we are able to help support individuals and communities across the country that are struggling to obtain basic levels of protection,” said Mike Clark, Division President at H&V. “Our new Nanoweb® FM media was designed specifically for general use in homemade face masks, and can be inserted in a face mask pocket or stitched into a disposable pleated mask.”
Consumers can purchase ready-to-sew face mask kits and Nanoweb FM media for homemade masks at www.sitnsewfabrics.com. One kit containing 4 masks will cost $24.95, and it is estimated to take 15 minutes to sew and assemble each mask.
Source: hollingsworth-vose.com & midwest-textiles.com
Camfil develops respiratory facemasks to support COVID-19 response in Sweden
In response to the Coronavirus pandemic, Camfil is designing and developing respiratory protection masks at its laboratory located in Trosa Tech Center in Sweden along with other parts of Swedish operations. The initiative was raised to meet the demand for respiratory protection masks and its testing.
Since such face masks are not part of regular manufacturing output, Camfil kicked off a large-scale internal initiative with R&D. Product development in the filter industry usually takes several months or even years, but due to urgent demand Camfil redeployed production lines while finding partners and suppliers who could help make the idea a reality in a short time. Along the way, Camfil consulted with health care organizations and partners to produce the best possible product and plan for execution.
Camfil’s face mask prototype has been tested in the lab and shows highly promising results, according to the company. It has also been tested, in different versions, by the staff at Karolinska University Hospital, who have been very positive towards the quality of the product. Production is now in full swing, and our goal is to provide Region Stockholm with 100,000 CamProtect, respiratory protection masks per week, initially.
Source: camfil.com
MIT professor explains test results showing some KN95 media provide filtration efficiency below 95% FDA mandate
As public health officials and others are working to secure personal protective equipment (PPE) to ensure the safety of health care providers and others working directly with COVID-19 patients. N95 respirators and facemasks, which are regulated by the U.S. government, have been in short supply, leading some entities and states to secure KN95 respirators as an alternative.

KN95 respirators are regulated by the Chinese government under specifications similar to N95 respirators in the U.S., and have been approved for emergency use in some circumstances by the FDA. (N95 respirators seal against the face and protect the wearer from small-particle aerosols in the environment. Face masks are not form-fitting but protect both the wearer and those around them from splashes and exchanges of large droplets.)
Recent tests conducted by the Massachusetts Manufacturing Emergency Response Team (M-ERT) in partnership with MIT have found that some of the KN95 filtration media in the states stockpile provides less than the 95% efficiency mandated by the FDA.
MIT Professor Gregory Rutledge participated in a Q&A to help explain the KN95 tests his lab conducted in cooperation with M-ERT.
Read the full story: http://news.mit.edu/2020/testing-kn95-respirators-rutledge-health-0430
KN95 test results: https://www.mass.gov/doc/kn95-respirator-test-results/download
Source: mit.edu
Sulzer provides liquid-liquid extraction in manufacturing of COVID-19 medicines
The urgency with which pharma businesses need to meet the rising demands of global healthcare production doesn’t provide sufficient time for them to significantly expand their operations by increasing their manufacturing footprint. For producers of antimalaria drugs, immunosuppressants and HIV medications, the pressure may be even higher, as these medicines are currently among the most promising treatments for the most critically ill coronavirus patients hospitalized in intensive care units.
Pharmaceutical ingredient manufacturer, Farmhispania, has found a way to address these challenges and produce more medicines suitable for coronavirus treatment. By working together with Sulzer, the leader in separation and mixing technology, the business will be able to ramp up its production capabilities in less than two weeks.
The two partners identified Sulzer’s ECR, a high-performance advanced extraction technology, as the most ideal solution. The separation system is particularly suitable for this project, as it can effectively handle a high flow rate, maximizing throughput.
Read the full story: https://www.sulzer.com/en/shared/news/220420-separation-technology-used-to-speed-up-fight-against-covid-19
Source: sulzer.com
Ahlstrom-Munksjö expands production of filtration media, targets 500 M facemasks in 2020 at Turin, Italy plant
Source: ahlstrom-munksjo.com
Xylem to issue grants to nonprofits working in COVID-19 impacted communities
Global water technology company Xylem, announced an expansion of its Partner Community Grants Program to further support customers and partners in the fight against COVID-19. To support community-level crisis responses, Xylem Watermark, the company’s corporate citizenship program, is inviting customers and partners worldwide to nominate nonprofit organizations in their communities to receive grant funding for COVID-19 responses.
The Watermark Partner Community Grants Program will accept nominations from Xylem customers, suppliers, and partners, anywhere in its global business network. Grants will be prioritized for nonprofits working in communities that are either currently heavily impacted by COVID-19, or are high-risk, low-resource locations.
Xylem will prioritize initiatives focused on the following areas:
- Infection mitigation: Efforts to prevent the spread and impact of COVID-19 cases
- Support and safety for critical services and front line personnel: Preparedness, mental health resources and training for front line workers at utilities, healthcare and critical care facilities
- Community sustainability and resilience initiatives: Including provision of critical life resources (i.e. food, water, medical care), small businesses support, youth support and education
- Projects aligned to Watermark priority focus areas: Including emergency response, Water, Sanitation and Health (WASH), water-related education and youth development programs
For more information on Xylem Watermark’s Community Engagement Grants, and to apply, visit https://www.xylem.com/en-us/watermark/covid-19-response/watermark-pc-grants-program/.
Source: xylem.com
Lenzing, Palmers form new company, Hygiene Austria, with a focus on protective mask production
Lenzing AG and Palmers Textil AG found “Hygiene Austria LP GmbH”, in which Lenzing AG holds 50.1% and Palmers Textil AG 49.9%. The newly founded company will start producing and selling protective masks for the domestic and European markets from May 2020.
Over the past few weeks, Lenzing AG and Palmers Textil AG have invested several million euros in a modern production infrastructure at the Wiener Neudorf location and secured the corresponding raw materials for protective masks production. In a first step, the company produces so-called mouth-nose protective masks (MNS) and surgical protective masks of class EN14683. Hygiene Austria LP GmbH plans to increase its capacities to over 25 million masks per month over the next few weeks and to expand this business geographically as well.
The demand for high-quality MNS and respiratory masks for medical personnel is increasing rapidly, and there is real competition on the international market for these products. In order to sustainably secure domestic supply now and in the future and to strengthen the business location, the two companies Lenzing AG and Palmers Textil AG have now set a milestone with their own competence center for hygiene based in Austria. Hygiene Austria LP GmbH thus makes a significant contribution to combating the Covid-19 pandemic and ensures the long-term supply of these critical goods in Austria in high quality.
“We decided to pool our knowledge, network and experience in a competence center for hygiene together with our partner Palmers. The aim of this joint venture is to provide the citizens of Austria and Europe with the best possible protection through locally manufactured, high-quality products,” says Stefan Doboczky, CEO of Lenzing AG. „Masks will continue to play an important role in our daily lives and we are proud that we were able to achieve our goal of an industrial production so quickly together with our partner Palmers.”
Read the full story: https://www.lenzing.com/newsroom/press-releases/press-release/lenzing-and-palmers-join-forces-to-fight-coronavirus
Source: lenzing.com
KARL MAYER proving machines to manufacture up to 250k facemasks per month
KARL MAYER is working with industry partners to ramp up facemask production, leveraging its double needle bar raschel machine to enable the production of roughly a quarter of a million masks per month. The masks are produced entirely without the need of any sewing work. Two models are available for the various demands.
Type 1 is produced at short notice and is suitable for everyday life. Due to their 3D shape, these masks have a tight fit and good wearing properties. They offer a convenient air exchange, a soft skin feeling, and prevent friction points on the ears, even after long-term use. The masks can be reused after their utilization. Simply wash and dry them, and the next application can start immediately.
Type 2 provides all the advantages of type 1, but it can be equipped with a replaceable nonwoven lining via a pocket. This increases the filtration capacity, at the same time ensuring minimum waste after use.
Regarding the filtering effect, the certification process is currently underway for a medical standard for both mask types.
To ensure a fast use of the face masks in Germany, KARL MAYER equipped a customer with the required machine technology and the instructions for the production of the masks. The mask manufacture will start by the end of April.
Once the installed KARL MAYER machine is running at full speed, it is possible to produce up to 400 masks per hour or 240,000 pieces per month. At the same time, KARL MAYER is working on reducing the production time for additional machines, so that capacity can be increased as quickly as possible. By mid-May we can achieve a production of up to half a million masks per month.
Source: karlmayer.com
Durst produces ‘community masks’ at Brixen headquarters
Durst, manufacturer of advanced digital printing and production technologies, is now producing “community masks” in its demo center at the Brixen headquarters in South Tyrol, Italy. The masks are produced in the first step for employees of Durst Group and sister company Alupress and afterwards the production capacity will also be available for other companies. The know-how for the production of the “community masks” will be made available to interested print service providers worldwide through its branches.
“At the beginning of April, our printing technologies for the label and packaging industry were classified as systemically relevant and we were able to go back into partial operation,” said Christoph Gamper, CEO and co-owner of Durst Group. “Now we will also use our textile printing and processing systems, which are located in the demo center for customer demonstrations, for the production of “community masks”. In line with our “pixel-to-output” strategy, production takes place digitally and fully automatically: in a web shop with a specially programmed editor, the masks can be individualized with graphics, images, texts, and our workflow software then sends the designed file directly to the printing machine and then the printed material is processed by a cutting system. The announcement of our initiative on social media alone has generated great demand and print service providers worldwide are adapting our concept and thus Durst technology for their productions.”
The “community masks” produced by Durst have a filter membrane that has a high filtration efficiency and at the same time is characterized by very good air permeability. The “community masks” have a three-layer structure, the polyester fleece textile materials are comfortable to wear and washable, the filter membrane can be disinfected with alcohol and reused.
Durst explicitly points out that this is not a protective equipment in accordance with VO (E) 2016/425 or a medical device in accordance with Directive 93/42 / EEC.
Durst has many years of experience with filter systems, as these take on a type of “cleaning function” in the printing press to filter out microparticles in the ink supply systems so that the print heads do not become clogged and are always ready for use. The filter membrane selected by Durst Development for the “community.
Source: durst-group.com
MANN+HUMMEL, Ford Motor Co. partner to produce respirators for COVID-19 response
MANN+HUMMEL announced it will collaborate with Ford Motor Company, one of its key automotive customers, to develop powered air purifier respirators in support of the COVID-19 response.
MANN+HUMMEL will supply the HEPA filter in the Ford-produced respirator, which will be worn by healthcare workers when treating COVID-19 patients. To date, MANN+HUMMEL produces, tests and ships 3,500 HEPA filter elements per day for this application, from their manufacturing sites in the USA and Germany. According to Ford, the automotive manufacturer is able to produce 100,000 respirators over the next two months.

The respirators are equipped with class H13 HEPA filters according to EN 1822 or the international norm ISO 29463. These filter classes are also used in operating rooms and in the pharmaceutical industry to reliably remove germs, viruses and microbiological contamination from the supply air. The European standard EN 1822 classifies HEPA filters, and evaluates them according to their performance at the MPPS (Most Penetrating Particle Size). The micro-glass fiber media used in pharmaceutical/healthcare applications typically has an MPPS close to 0.2 μm. Respectively, filter class H13 has an efficiency of at least 99.95 percent.
With the acquisition of the European Vokes Air Group in 2014, Tri-Dim Filter Corporation in 2018 and Hardy Filtration in 2019, MANN+HUMMEL added almost 90 years of leading air filtration and cleanroom technology expertise as well as a state of the art HVAC and HEPA filtration portfolio to its unmatched portfolio of automotive and industrial filtration products and services.
Source: mann-hummel.com
Ahlstrom-Munksjö tapped by French government to produce material for 100M facemasks
Ahlstrom-Munksjö has developed a facemask material as part of the Resilience project, administrated by the French Government. During May, the company will produce material equivalent to 100 million facemasks for civil servants in contact with the public such as police, prison administration and social workers as well as companies active in essential sectors e.g. food, energy, water and waste.
In accordance to the newly implemented guidelines issued by the French National Agency for the Safety of Medicines and Health Products (ANSM), Ahlstrom-Munksjö’s products Reliance SMS 200, Reliance SMS 300, Reliance Dextex 200, and Reliance Dextex 300 have been declared compatible with the French requirements for face masks used by civil servants in contact with the public. The material is typically used for the manufacturing of sterilization wraps for surgical instruments. Reliance SMS 200 and Reliance SMS 300 have also been tested compatible with the European standard EN 14683, meeting the performance criteria of surgical masks.
“The attainment of this long-term work for the selection of our products represents a moment of collective pride at Ahlstrom-Munksjö. Our Medical business serves the medical device market worldwide all year round, and our agility and ability to innovate makes us an ideal partner in critical situations,” says Lionel Bonte, Vice President of Medical business at Ahlstrom-Munksjö.
In France, Ahlstrom-Munksjö has some 1,600 employees, eight productions sites, two global R&D centers, and a sales office.
Source: ahlstrom-munksjo.com/
Sonobond Ultrasonics granted COVID-19 waiver, continues to produce ultrasonic bonding technology for PPE & needed medical supplies
Sonobond Ultrasonics has been granted a waiver from Pennsylvania’s COVID-19 Orders so it can continue producing equipment that assembles medical supplies. The company’s textile assembly machines help to increase production speed and create barrier seams that satisfy regulatory requirements for face masks, medical gowns and other nonwoven textile medical supplies needed during the COVID-19 pandemic.
The machinery uses high-frequency ultrasonic waves to produce sealed edges and secure barrier seams.
Learn more: https://www.filtnews.com/sonobond-ultrasonics-machinery-creates-barrier-seams-the-meet-covid-19-requirements/
Source: sonobondultrasonics.com
Superior Felt & Filtration ramps up PPE production, readies 75K sq.ft. cleanroom to support COVID-19 response
In response to the COVID-19 pandemic, Superior Felt & Filtration is announcing immediate expansion to meet the global surge in demand for Personal Protective Equipment and medical filtration needs. In June 2020, the company will expand into an additional 75,000 sq. ft. state-of-the-art cleanroom facility. Superior has also recently invested in five new pieces of manufacturing equipment that will allow more capacity for collating and slitting of media, as well as the manufacturing of sub-component filters and other lifesaving nonwoven technologies.

In ramping up in the face of the COVID-19 pandemic, Superior Felt & Filtration, LLC has tripled its capacity over the last two months, which will continue to grow with the coming facility expansion and new equipment investment.
Based out of McHenry, IL, Superior is an essential subcomponent manufacturer supporting the disposable surgical hood, N95 mask component, BFE/VFE and ventilator filter markets. Meeting the immediate medical filtration needs of the U.S. government and its partners under the Defense Production Act, Superior has a global reach, exporting lifesaving filter technology across six continents.
As a strategic partner with Hollingsworth and Vose and utilizing Technostat, an electrostatic filter media, Superior has formed partnerships with vital companies in the fight against COVID-19, including Honeywell, GM, Ventec and Phillips.
Source: superiorfelt.com
NatureWorks, NWI partner to produce spunbond-based nonwoven material for N95 facemasks
As the world faces a critical shortage of personal protective equipment (PPE) for medical workers confronting the COVID-19 crisis, a long-standing partnership between NatureWorks and the Nonwovens Institute (NWI) at North Carolina State University (NC State) has resulted in a new spunbond nonwoven technology enabling the production of at least 10 million additional N95 surgical masks. NWI has converted the use of its research and training pilot production line to produce the face mask materials, and NatureWorks has donated the Ingeo resin needed to produce the spunbond material.
“Because of the COVID-19 crisis, we took the spunbond technology and created a new generation of unique filters that have excellent filtering capability without needing to be charged, meaning they can potentially be reused after cleaning with peroxide, or an alcohol solution,” says Behnam Pourdeyhimi, executive director of NWI, Wilson College of Textiles associate dean for industry research. and extension, and William A. Klopman distinguished professor. “Because these materials are also strong they can be cut and sewn by traditional techniques.”
The new nonwoven fabric is a bicomponent fiber made of Ingeo biopolymer (PLA) and polypropylene (PP), providing significant strength and bulk with equal effectiveness in filtration. Additionally, Ingeo improves the productivity of the spunbond process by at least 30%. Leveraging these benefits, NWI’s pilot line can produce enough material to make 2 million masks per week.
Read the full story: https://www.natureworksllc.com/News-and-Events/Press-Releases/2020/2020-4-22-NWI-N95-masks-release
Source: natureworksllc.com & thenonwovensinstitute.com
Achieving US self-sufficiency on meltblown fabric for facemasks
The current COVID-19 pandemic has exposed the reliance by the U.S. on Asian supply chains for much needed Personal Protective Equipment essential to deal with the situation. Of primary importance is the supply of medical/surgical facemasks and gowns for healthcare workers. INDA, Association of the Nonwoven Fabrics Industry, is currently assisting many government groups trying to address this issue. INDA’s president, Dave Rousse, outlines what would be needed for the U.S. to become self-sufficient in the key material area impacting face masks so we do not encounter shortages in times of critical need.
Read the full story: https://fiberjournal.com/achieving-us-self-sufficiency-on-meltblown-fabric-for-facemasks/
Berry outlines COVID-19 actions, launches new media for facemask materials
Berry Global announced strategic initiatives to increase production of face mask materials. The initiatives include the company designating additional capacity for the production of facemask materials in North America and introducing a new material for facemasks in Europe. With demand outpacing current supply for facemask filter media, the product development team at Berry has responded to deliver innovative solutions in a matter of weeks to support the demand. These solutions include pivoting existing manufacturing assets and creating alternative materials for facemasks.
To address the ever increasing demand for facemask material in the United States, Berry has expanded its proprietary Meltex platform to add meltblown capacity in Waynesboro, Virginia. This capacity was converted from a pilot line into one which provides full commercial output. The line will make meltblown materials, which will ultimately be used in surgical-grade face masks along with N95 and N99 respirators. This added capacity will support the manufacturing of approximately 200 million facemasks annually, according to the company.
In addition to its Waynesboro facility, Berry has a number of North American nonwovens manufacturing facilities which are rapidly producing materials that help protect against the spread of COVID-19. The added capacity in Waynesboro is complementary to the company’s existing portfolio, producing incremental output surgical-grade materials serving the North American market.
Berry also launched an extension to its Synergex range of products, Synergex ONE, a new media for facemask applications. Developed to initially meet the new facemask categories for general population, the aim is to quickly bring the media up to EN 14683:2019 standards for surgical masks. The newly introduced Synergex ONE provides a multilayer nonwoven composite product in a single sheet, as an alternative to traditional facemask layer structures. This new material will be manufactured in Europe and serve the European market and is available immediately.
Source: berryglobal.com
Ahlstrom-Munksjö materials validated by French Government for facemask production
In accordance to the newly implemented guidelines issued by the French National Agency for the Safety of Medicines and Health Products (ANSM) aimed at providing policies that help expand the availability of face masks for non-sanitary use, Ahlstrom-Munksjö has received necessary certification for the use of its Reliance SMS 200, Reliance SMS 300, Reliance® Dextex 200 and Reliance Dextex 300 materials for facemask production. Typically used for the manufacturing of sterilization wraps for surgical instruments, these materials have been deemed to meet the requirements of a category 1 mask type for use by professionals in contact with the public. In addition, Reliance SMS 200 and Reliance SMS 300 have been shown to be compatible to the performance criteria of surgical masks.
The validation was acknowledged following a series of tests conducted by the Direction Générale de l’Armement (DGA), the French Government Defense procurement and technology agency. Testing confirmed the performance compatibility of the Reliance SMS material with surgical facemask types thanks to its breathability and bacterial filtration efficiency, whereas Reliance Dextex proved to ensure permeability to air and to perform in protection barrier efficiency at a level compatible to the category 1 face masks currently in use.
Reliance SMS 200, Reliance SMS 300, Reliance Dextex 200 and Reliance Dextex 300 manufactured at the Medical business plants in Windsor Locks, United States and Mundra, India and converted at the site in Pont Audemer, France, have already been supplied to hospitals across France, to the French Government and distributors.
Source: ahlstrom-munksjo.com
Johns Manville starts production of filtration media for facemasks at Richland, Mississippi plant
The Johns Manville plant in Richland, Mississippi, has started production to make nonwoven filtration media that will be used to create needed facemasks to help stop the spread of COVID-19.
Most nonwoven production of face masks was abandoned in the U.S. many years ago and moved to Asia. Given the shortage of face masks in the U.S. and Europe, JM’s Engineered Products business decided to build on its existing capabilities and help fill the market demand.
JM media meets or exceeds Level 1 BFE 95% (Bacterial Filtration Efficiency) and VFE (Viral Filtration Efficiency) requirements. These results were verified by an FDA-registered national laboratory.
The JM plant in Richland employs about 50 people and produces a variety of filtration products for various air and liquid applications using polypropylene and polyester meltblown technology. Richland’s meltblown filtration media can be found in numerous industrial, automotive, consumer products and FDA-approved food and healthcare applications.
Source: jm.com
Researchers develop self-sterilizing air filter that could be used for N95 and other facemasks
With the worldwide focus on coronavirus prevention and transmission, a new type of air-filter that self-sterilizes and decontaminates is being developed at Ben-Gurion University of the Negev in Beer-Sheva, Israel.
The new nanotechnology is based on laser-induced graphene (LIG) water filters that eliminate viruses and bacteria in water. This new concept, engineered for air-filtration could be used in air filters in heating, ventilation and air-conditioning (HVAC) systems or integrated into face masks for a self-sterilizing effect. Most masks will become contaminated during usage, including the N95 respirator mask, and if not properly used or handled, becomes a contamination risk.

LIG is a microporous graphene foam that can be generated on many types of materials. LIG on water filters provide an active protection with simultaneous contamination removal and disinfection. The LIG is already resistant to bacteria and actively kills microbes and viruses using a low-level electric current from a power source. The researchers envision the two-fold protective system applied to air-filtration.
“The bacterial-resistant graphene surface protects against microorganisms so they can’t multiply, while the microbes trapped in the filter are eliminated by electrical effects.” says inventor Dr. Chris Arnusch, senior lecturer and researcher at the BGU Zuckerberg Institute for Water Research, part of the Jacob Blaustein Institutes for Desert Research.
The material can be completely sterilized by electrical current, thus an LIG air filter has the potential to be combined with state-of-the-art air filtration such as HEPA filters. The filters could add an active layer of protection, as well as prolong the lifetime of the expensive HEPA technology. As a result, hospitals, cars, buildings and public transport could all become safer spaces.
If such a material is incorporated into a mask a higher level of protection for medical providers, as well as the general population, could be possible, according to the researchers.
Source: aabgu.org
RKW joins coalition of manufacturers to produce FFP-2 protective facemasks
In response to the COVID-19 pandemic, RKW Group, Sporlastic, the Gherzi Consulting Group and others have formed the “FIGHT” consortium to jointly produce FFP-2 type protective masks.
RKW is participating in the production of FFP-2 protective masks by producing a laminate made of spunbonded nonwoven fabric at its Gronau, Germany facility. Production has been running at full speed for a few days now, with the aim of achieving a weekly capacity of around 750,000 masks.
The RKW spunbonded nonwoven is processed in Gronau into a laminate with meltblown material that meets the high demands on the filter material for FFP-2 masks. It must capture at least 94 percent of the particles in the air down to a size of 0.6 micrometers. The COVID-19 coronavirus is bound in water droplets averaging around 1 micrometer in size.
The first production batch will be delivered to the first client, the Ministry of Social Affairs and Integration of Germany’s federal state of Baden-Württemberg. Further public sector clients are currently in negotiation with the consortium.
Source: rkw-group.com
Sandler AG to start new meltblown line for filter media in Q3 2020
Sandler AG, a producer of meltblown nonwoven materials for filtration applications, announced it will be starting a new meltblown production line in the middle of the third quarter of 2020 at its nonwoven facility in Perry, Georgia. The annual output of the new line will support the manufacture of up to 800 million facemasks to aid in the COVID-19 response. With this capacity expansion, the company intends to contribute to rapidly counteracting the severe shortage of filter materials for respirator masks and to establishing complete value chains.
Source: sandler.de
NC State’s Nonwovens Institute calls for collaborators in making facemask materials and masks for COVID-19 response
The Nonwovens Institute (NWI) at North Carolina State University is responding to the shortage in capacity for making filtration media and converting it to make facemasks by opening up its meltblown and spunbond nonwoven-making facilities. NWI is utilizing its expertise to produce specially designed fabrics that can be delivered to manufacturers in the U.S. to assemble facemasks, and it is also investing in new converting equipment to locally assemble ready-to-use facemasks for healthcare workers, as well as for public use.

NWI is also seeking collaborators, partners, suppliers, and customers to engage in the response by contributing money, equipment and materials.
Need area:
- Cash Donation (online, check, wire transfer)
- Equipment & Materials Donation
What’s needed?
- Investment support to scale-up nonwoven production, invest in new converting equipment, and increase capacity.
- Equipment for production and maintenance, e.g., Reicofil Spare Parts for Production, nonwoven belts, SB and MB resins (polypropylene & PLA), forklifts, and product handling and storage equipment.
Impact:
- Accelerate time to full production of affordable facemasks that make a difference.
- Ensures production reliability and resin supply for production.
Questions about this initiative can be directed to nonwovens@ncsu.edu.
To learn more about facemask technology types and applications, Behnam Pourdeyhimi, Ph.D., NWI Executive Director, has posted a detailed description here.
Source: thenonwovensinstitute.com
Filtration Group Industrial converts production to focus on breathing masks
Filtration Group Industrial has converted a part of its production to breathing masks. Approximately 30,000 masks will be produced weekly from the company’s production facility in Öhringen, Germany.
“In a first step, we will equip our employees with our own breathing masks. In this way we can ensure that production can continue for our core business and at the same time that we take appropriate account of health protection,” said Gunnar Halden, president of Filtration Group Industrial. “We have never had a change in production at this speed before. I am proud of what we have achieved together in a very short time. ”

After providing its own workforce with breathing masks, local companies and institutions of public interest, as well as private individuals, will be supplied. The Filtration Group would like to donate the first 10,000 masks to public institutions at its production sites. By producing breathing masks for private use, Filtration Group Industrial said it is aiming to help ensure that higher-quality, medically certified protective masks according to the FFP2 and FFP3 standard can continue to be used in hospitals and medical centers.
Source: https://industrial.filtrationgroup.com/
Coffee filter giant, Melitta, retools its production to manufacture up to 1M facemasks per day
As a contribution to supplying urgently needed breathing masks in response to the COVID-19 pandemic, coffee filter giant, the Melitta Group, is converting parts of its production to help fill the void. In the production of Melitta filter bags, breathing masks are now also being manufactured industrially and in large numbers at the company plant in Minden, Germany.

“With our production capacities, we are able to produce very large quantities of breathing masks in the shortest possible time,” says Jero Bentz, member of the Melitta management team. “For decades, our company has specialized in developing and producing other filter materials such as vacuum cleaner bags and other special industrial papers such as nonwoven wallpaper, primary materials for air filters — also for the medical sector — in addition to our filter bags.”
The result is a breathing mask in the form of Melitta’s filter bags, consisting of a three-layer fleece, which contains a meltblown layer that already fulfills the surgical mask standard according to EN14683 with a bacterial filtration efficiency (BFE)> 98%. In the first step, these masks are attached with a clip or rubber bands. The further development of the masks is planned in further expansion steps, with production of FFP2 and FFP3 masks also under development.
Melitta has already produced the first million masks. The existing capacities are expected to produce up to one million masks per day shortly. These quantities can be successively increased many times over, according to Melitta, provided the raw materials are sufficiently available. In addition to Germany, the Melitta Group also plans to use the existing production capacities in the U.S. and Brazil to manufacture breathing masks for North and South America.
Source: melitta-group.com
Webinar Series: Nonwoven market, products and supply in the world of COVID-19
INDA, Association of the Nonwoven Fabrics Industry, will present the first in a three-part webinar series focused on the role nonwoven materials are playing in the COVID-19 response, Tuesday, April 14, 2020, 2:00 pm–2:50 pm EST.
Part I in the series, titled “Structure, Effectiveness and Differentiation of COVID-19 Wipes & Masks,” will be presented by Chris Plotz, Director of Education & Technical Affairs, for INDA. The webinar will cover physical and chemical characteristics of disinfecting nonwoven hand wipes, hard surface wipes, and facemask technology for COVID-19 response in work, home, and personal care applications. Highlighted topics will include:
- Face mask: Differentiation and filtration efficacies
- Wipes: Dry and wet
- Chemistries: Types and effectiveness for hand, skin, and hard surface sanitizers
- Nonwovens best protection practices and new developments
Plotz is a business leader with 19 years of technical nonwovens related experience in global product management and product development. At INDA, Plotz directs, oversees, and expands education and training programs for all levels of industry members, manages the international harmonized standards activities, Product Stewardship activities, Technical Advisory Board, and key services areas that INDA operates for its members.
Subsequent webinars in the series include:
- “Absorbent Hygiene Marketplace During COVID-19: Disruption, Risks, & Opportunities,” Svetlana Uduslivaia, Head of Research, Euromonitor International
- “Increasing Nonwovens Materials to Respond to COVID-19 Crisis,” Brad Kalil, Director of Market Intelligence and Economic Insights, INDA
Register for the webinar series at: https://imisw.inda.org/store/SearchResults.aspx?searchterm=webinar&searchoption=ALL
Source: https://www.inda.org/
* INDA owns International Filtration News.
Celeros Flow Technology to donate 50K surgical masks
Celeros Flow Technology, a newly formed spinoff of SPX Flow, has committed to donate 50,000 surgical masks to community organizations in the Charlotte, NC area. These masks will be distributed locally through the company’s operating locations.
Celeros Flow Technology brands include Clyde Union Pumps, M&J Valve, GD Engineering, Plenty, Copes Vulcan, Dollinger, OFM Services, Airpel and Vokes.
Source: celerosft.com
McIlvaine touts reusable facemasks as alternative to social distancing
According to a report by McIlvaine Company, social distancing needs to be redefined from the common concept of staying six feet apart of other individuals or the space they have occupied in the last 60 seconds. This current definition creates great barriers to normal life. The safety of social distancing can be alternatively provided with a program that will have comfort and social acceptance along with affordability. This is now possible because of new filtration media, testing, decontamination and other technology innovations.
It starts with the fact that a washable highly efficient mask can be provided.
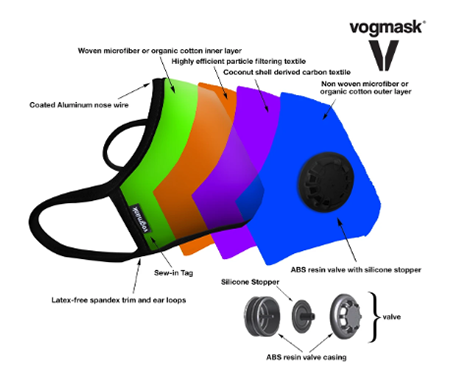
There are retail stores in China that sell nothing but these masks with many fashionable varieties. With an integrated valve, they are both comfortable and stylish. They are also washable. These are $30 masks, which if worn 60 times, would cost $.50 per wearing. There are less expensive designs available as well. Chinese companies are also producing washable N95 masks with and without the valve feature. Over the last few months, the production these masks has expanded to several million per day, and since they are washable the cost per wearing is as low as 10 cents.
Source: mcilvainecompany.com
Read the full story: http://home.mcilvainecompany.com/index.php/other-services/free-news/news-releases/47-news/1573-nr2569
Related story: https://www.filtnews.com/as-coronavirus-surges-filtration-questions-arise/
ANDRITZ introduces high-speed facemask converting line
In the first configuration, this new converting line will be able to produce masks for surgical/medical applications; other mask types – like N95/FFP2 – are currently being evaluated.
The new ANDRITZ D-Tech Face Mask line produces and laminates three or more layers of fabrics (spunbond, meltblown, thermo-bonded nonwovens and others). It comprises unwinding and guiding units for nonwoven webs, cutting and positioning devices for the metal nose bar, an edge welding and cutting unit, a 90 degree rotation process, as well as positioning and welding of the ear loop elastics.
The line has a speed of up to 110 m/min and is able to produce up to 750,000 facemasks per day, according to ANDRITZ. There are also different packaging options available: products can be packed in bags by an automatic flow wrapping machine or in cardboard boxes by an automatic cartoner.
Source: andritz.com
Sinopec Yizheng Chemical Fibre Co. launches first of 12 planned meltblown lines
Sinopec Yizheng Chemical Fibre Co (YCFC) successfully launched its first melt-blown non-woven production line on March 29 in Yizheng, Jiangsu province, which was manufactured by Shaoyang Textile Machinery Co, affiliated to Sinomach’s subsidiary China Hi-Tech Group Corporation (CHTC). Shaoyang Textile Machinery succeeded in reducing the lead time to a month and delivered the production line 13 days earlier than the date required by the State-owned Assets Supervision and Administration Commission of the State Council (SASAC).

As the filter layer of a surgical mask, the meltblown nonwoven material is an essential raw component in mask production. Mask production lines are expanding rapidly across the country as an urgent part of pandemic prevention and control, and a large number of manufacturers are facing a shortage of raw materials.
Shaoyang Textile Machinery will supply 12 meltblown nonwoven production lines in total to YCFC. When all 12 lines are operational, YCFC will have a daily output of 12 tons of N95 melt-blown non-woven or 18 tons of surgical mask materials, and be able to increase production of disposable surgical masks by 18 million pieces a day.
Source: sinopec.com
Web Industries subsidiary, Omega Systèmes, shipping 900K French Directorate-approved facemasks
Omega Systèmes, a Web Industries company, is shipping 900,000 personal facemasks approved by the French General Directorate of Armament to the Ministère des Armées for distribution to food manufacturers, supermarkets and nursing homes conducting business in France.
Obtaining the French General Directorate of Armament approval for mask production called for Omega to pass a series of tests carried out in accordance with stringent Armament specifications, including those for wearer protection.
The announcement comes at a time when demand for the facemasks is high throughout France. Prime Minister Édouard Philippe has extended COVID-19 containment measures until April 15th, as the number of infections in France exceeds 81,000 with more than 10,800 deaths in hospitals. The masks are being supplied by the Ministèred Armées to businesses whose personnel are at a high risk of contamination, such as those working in the food sector and facilities housing the elderly. These single-use masks can be worn for up to four hours before being changed and offer a 96% protection rate.
Source: webindustries.com
Youngstown State researchers develop 3D-printed filtration cartridge for medical facemasks
A team of Youngstown State University faculty, working with physicians, nurses and respiratory therapists at Mercy Health Youngstown (Ohio), have designed a filtration cartridge that could help address the shortage of personal protection equipment in the ongoing fight against the coronavirus.

Youngstown State University engineering faculty have developed a 3D-printed filtration cartridge that will fit on protective masks of medical workers on the front lines of the COVID-19 pandemic.
The 3D-printed device, under review by the National Institutes of Health’s Print Exchange, creates a reusable, adaptable filter cartridge that can be attached to commercially available protective masks used in healthcare settings.
“The project addresses the need by doctors, nurses and other frontline medical providers for a readily-available substitute for N95 face masks during the pandemic,” said Darrell Wallace, YSU professor and program coordinator for Manufacturing Engineering.
Read the full story: https://ysu.edu/news/faculty-design-could-help-coronavirus-fight
Source: ysu.edu
What You Need to Know About Air Purifiers and the Coronavirus
Running an air purifier at home can be a good idea anytime, to help filter out indoor allergens and pollutants like fumes from cooking and cleaning products. Especially now, when so many people are stuck indoors 24/7 because of the pandemic.
But can an air purifier prevent COVID-19 by capturing virus particles that could be traveling in the air?
We spoke with air quality and virology experts, and asked CR’s own experts to weigh in. The consensus is that while air purifiers probably don’t offer much protection in most circumstances, they may be worthwhile in a few specific ones.
Read the full story: https://www.consumerreports.org/air-purifiers/what-to-know-about-air-purifiers-and-coronavirus/
Source: consumerreports.org
Ahlstrom-Munksjö ramps up medical fabrics production to provide up to 30 million face masks per month
Responding to the COVID-19 pandemic, Ahlstrom-Munksjö has increased capacity across its entire protective medical fabrics product portfolio. The exponential spread of the COVID-19 virus around the world has caused an exceptional situation, creating strong demand for healthcare goods in general, and specifically for protective medical products that are made from the company’s fabrics. Ahlstrom-Munksjö is also expanding manufacturing of fabrics production lines normally used for producing other fiber-based materials.
In the beginning of March, the company expanded the production of face mask materials to a fine fiber line normally used for industrial filtration materials at the Turin plant, Italy. The technology is unique, generating good protection and breathability for medical use, and approved as a surgical mask. The advantage of this technology compared to the commonly available electrostatically charged material is its durability. Protection efficiency of electrostatically charged materials is lost overtime when humidity gets in contact with the filter media, whereas protection efficiency of a mechanical filter media remains intact over time. First orders were received in the middle of March, and Ahlstrom-Munksjö is now targeting to ramp up fabrics production for more than 20 million face masks per month.
In April, Ahlstrom-Munksjö is expanding the production of face mask materials to a line normally used for industrial filtration materials at the Tampere plant in Finland. Currently the material is well suited for lighter fabric face masks in civil activities. Droplets test shows that the media has efficiency above 90% at 3 microns and above 85% at 1 micron, which is close to a surgical face mask requirement. Development continues to meet the requirements of material for surgical masks for medical use. Considering the ongoing production, the Tampere plant can deliver fabrics for around 10 million face masks per month on a short notice.
Source: ahlstrom-munksjo.com
Reifenhäuser calls for the creation of industrial supply chains for face masks in Europe
Reifenhäuser Reicofil recently opened its R&D facility to the manufacture of nonwoven fabrics for face masks and medical protective apparel to support the COVID-19 response. However, the company says the utilization of this material is under-performing, with only small-scale, manual conversion of the material into face masks for end use.
“Within the last two weeks, many initiatives have been founded that sew masks by hand. Thus, at least small production capacities have been established in Germany within a very short time, which now want to be supplied,” said Bernd Kunze, Ph.D., managing director of Reifenhäuser Reicofil. “From the many inquiries we have very consciously selected those initiatives that work efficiently and deliver masks quickly and reliably first to where they are most urgently needed.”
For Reifenhäuser, however, manually operating converters remains only a partial success, as the quantities produced in this way are only a small amount of the overall demand for medical protective clothing. Likewise, the company notes that if the general population is to be supplied with masks, the demand would rise into the billions. To actually solve the problem, the company said Germany and Europe need to establish their own industrial production sites with closed European supply chains and decisive political action. The aim must be to supply Europe with protective material independently and competitively, both now and in the long term.
“We need a strategic production reserve for medical protective clothing in Europe,” said Bernd Reifenhäuser, CEO of the Reifenhäuser Group. “We have to quickly build up the machine capacity for the industrial production of masks in high volumes, but at the same time, the corresponding capacities for the production of the necessary high-quality nonwovens in Europe must be created.”
Source: reifenhauser.com
Porvair adapts manufacturing processes to provide filtration technology for COVID-19 response
Porvair has adapted its manufacturing processes based on calls for businesses to support in the production and supply of ventilators and ventilator components to support COVID-19 response. The company’s actions include:
Ventilator and breathing apparatus:
- Segensworth Division has been selected to support Project Oyster (the British Consortium of carmakers, Formula One teams, and airplane manufacturers) which have been urged to build 10,000 ventilators. Porvair will be supplying flat discs to be used as air filters, to protect against dust ingestion.
- Wrexham Division is manufacturing filter components for several of the organisations who are now building breathing apparatus to hospitals.
- Caribou Division is manufacturing and supplying porous metal parts for use within ventilators, clinical analysers and respirators.
Medical testing:
- Sciences Division has increased production of millions of pipette tips to be used in the United States for their Covid-19 testing kits, as well as supplying consumables for dozens of US testing laboratories.
- Supplied bulk filtration media to one of our German distributors to be manufactured into filters for the German Covid-19 testing program.
- Wrexham Division is undertaking a rapid development project to use existing DNA extraction products and use the technology to design and manufacture RNA extraction kits.
Pharmaceutical production
- Supplying chromatography bed supports and pharmaceutical grade water to pharmaceutical customers whose production is increasing.
- Seal Analytical are supplying water quality testing kits and general industrial filters to pharmaceutical and chemical reagent customers for their requirements.
Source: porvairfiltration.com
Related article:
As coronavirus surges, filtration questions arise
PFAFF Industrial publishes tutorials on mask making via high-speed seamer and via ultrasonic bonding
PFAFF Industrial offers two video tutorials showing how facemasks are made.
One video showcases facemask making via a high-speed seamer.
The other showscases facemask making via ultrasonic bonding.
Cummins, DuPont working on alternative material for N95 respirator masks
As the COVID-19 pandemic spreads across the globe, Cummins Inc. and DuPont are helping address the nation’s shortage of N95 respirator masks. Cummins’ NanoNet® and NanoForce® Media technology, which uses DuPont’s Hybrid Membrane Technology (HMT), can typically be found in air, fuel and lube filtration products used in heavy-duty diesel engines to prevent long-term engine wear, but also can be used in the N95 respirator masks worn by healthcare professionals to filter harmful airborne particles that can spread COVID-19.
The need for N95 masks has skyrocketed in recent weeks in response to the COVID-19 pandemic. Many of the world’s leading mask manufacturers are in need of the critical materials to assemble the mask and are struggling to meet demand.
“Cummins is re-evaluating our supply base and manufacturing capabilities to identify how we can support our healthcare professionals who rely on critical personal protective equipment to do their jobs,” said Amy Davis, Vice President of Cummins Filtration. “Our NanoNet® Media can fill a key supply void and help address the mask shortage facing the United States and other countries around the world.”
The first mask prototypes using Cummins’ donated media were assembled by University of Minnesota teams in March as part of an initiative to provide masks to M Health Fairview and other Minneapolis-based healthcare systems. As the COVID-19 outbreak escalated, the University of Minnesota realized their supply of N95 masks to protect healthcare workers would potentially run out in a matter of weeks. To address this challenge, a team of designers, engineers, chemists, surgeons, anesthesiologist and apparel and clothing experts from the University of Minnesota’s Institute for Engineering in Medicine; Medical School; College of Design; College of Science and Engineering; and Center for Filtration Research Consortium (CFR) came together to address this projected shortage of critical personal protective equipment.
“The first thing we recognized from our experts in the Center for Filtration Research, who work directly with Cummins, is that not all filtration materials are created equal and that the Cummins material is an excellent alternative,” said Jakub Tolar, Campus Health Officer and Medical School Dean at the University of Minnesota. “We are tremendously grateful for the generous donation from Cummins of their filtration materials toward our mask effort. Since the arrival of the filtration media, we have been able to make rapid progress, and we now believe we have several viable mask options, including both a disposable and re-usable option. These designs show real promise in keeping our healthcare workers safe should standard medical supplies of N95 masks no longer be available,” continued Tolar.
DuPont’s Hybrid Membrane Technology goes beyond the limits of traditional semi-porous or nonwoven membranes for air and liquid filtration. Made using a proprietary spinning process, the hybrid technology materials are comprised of continuous sub-micron fibers. The end result is a “membrane-like” sheet structure that balances breathability and high filtration efficiency of particulates.
While products featuring Cummins’ media will need to be vetted and approved by the National Institute for Occupational Safety and Health (NIOSH), the company is preparing to do its part to help relieve the burden facing the healthcare industry. “We’re working as quickly as possible with healthcare regulators and other partners to help certify products with our materials, and prepare our manufacturing facilities to meet demand,” said Davis.
Mask manufacturers interested in learning more about Cummins’ media technology can visit cumminsfiltration.com/respiratormedia.
Source: cumminsfiltration.com
ISAIC partners with area organizations to produce millions of medical- & surgical-grade masks in Michigan
The Industrial Sewing and Innovation Center (ISAIC) is working with the City of Detroit, the Michigan Economic Development Corp., Carhartt, Rock Family of Companies to mobilize local apparel manufacturers to produce standardized, centralized PPE and automate mass production of pleated surgical masks. The initiative is expected to produce millions of pleated medical grade masks and thousands of sewn surgical masks and isolation gowns.
Under this regional initiative, the organizations are making mask and gown kits with standardized product specifications, created in consultation with area hospitals, to be dispersed to local manufacturing partners for production. ISAIC will administer orders, control inventory and handle distribution to hospitals and other medical facilities.
If you are a hospital or medical facility in need of PPE, click here.
If you are a local sewing specialist or manufacturer and want to get involved, click here.
Source: isaic.org
Berry contracts with Reifenhäuser Reicofil for new meltblown line in France to produce respirator materials
Reifenhäuser Reicofil and Berry Global Group have signed a contract for a meltblown line to produce filter material for FFP2 (N95) and FFP3 (N99) respirators. The plant is scheduled to be delivered in June. In order to support the fight against the coronavirus, Reifenhäuser Reicofil has reduced the delivery time of the line by one third, to 3.5 months.
The 1.6 m wide Reicofil meltblown line is equipped with a technology for electrostatic charging for filter materials, a key component of face mask filtration. The plant will bring an additional annual capacity of 550 tonnes of N95 material and 365 tonnes of N99 material, respectively. serving the EMEA market.
The plant will be installed in France.
“We are pleased that the Berry Group, the world’s largest producer of nonwovens, is investing in new capacities for urgently needed, high-quality filter material,” said Bernd Kunze, Ph.D., CEO of Reifenhäuser Reicofil. “The demand for these materials is currently enormous and will, in our opinion, remain so in the future. We will provide all producers, who want to get involved in the fight against COVID-19, with the best possible support for rapid project realization.”
Source: reifenhauser.com & berryglobal.com
NC State’s Nonwovens Institute to employ novel material for production of masks and mask filters
NC State’s Nonwovens Institute (NWI) is using its two research and training pilot production lines to produce face mask materials that will be used to protect medical workers supporting the COVID-19 response.
N95 respirators and surgical masks are generally a sandwich of one or two common nonwoven layers – so-called spunbond layers that provide mask shape and protect the inner filtration layer – combined with a layer of nonwoven meltblown material that serves as the filtration layer and captures microscopic unwanted particles like viruses and bacteria.
But because of the current critical need for masks caused by COVID-19, the NWI team created a new spunbond material that can serve as an effective filter without the need for a meltblown filtration layer. The unique fabric is composed of two different polymer materials that are combined to make a single fiber with significant strength and bulk – and that shows effectiveness in filtration similar to current materials used.
“Because of the COVID-19 crisis, we took the spunbond technology and created a new generation of unique filters that have excellent filtering capability and can potentially be reused after cleaning with peroxide, or potentially alcohol solution,” said Behnam Pourdeyhimi, executive director of NWI, Wilson College of Textiles associate dean for industry research and extension and William A. Klopman Distinguished Professor. “Because these materials are strong, unlike classical meltblown filters, they can also be cut and sewn by traditional techniques.”
Typically, one meter of spunbond material provides enough material for about 20 to 25 masks when using the current designs, Pourdeyhimi said. One of the NWI’s production lines started producing 2,000 meters of spunbond material per hour, with the potential to create some 20,000 meters of spunbond material in a day. NC State is currently in discussion with industrial partners to make masks with the material.
NWI’s other production line is a meltblowing pilot line that will make the classical meltblown material for N95 masks and surgical masks.
“We created a recipe for the production of classical N95 respirator materials and will ship those materials out for industrial partners to convert these into respirators,” Pourdeyhimi said.
The meltblown material takes a bit more time to produce; Pourdeyhimi estimates that his production line can make about 12,000 meters of material in one work shift.
Thanks to support from across the university, Pourdeyhimi says that NC State has ordered machines that will allow the NWI to make surgical masks in its Centennial Campus facilities. Those machines should arrive in the next month.
“We will set these machines up and take our own materials and convert them into masks and provide them to local communities,” Pourdeyhimi said.
Source: thenonwovensinstitute.com
NCTO calls on U.S. to abandon 90-day tariff deferral on textile imports
The National Council of Textile Organizations (NCTO), an association representing U.S. textiles from fiber through finished products, issued a statement from NCTO President and CEO Kim Glas today in response to the U.S. administration’s plan to institute a 90-day deferral on MFN tariffs, as reported by numerous press outlets.
The reported plan being pushed by the importing and retailing industries would defer certain tariffs, including those on finished apparel products. It is an ill-advised policy that will hurt the U.S. textile industry at the very time it is answering the call of the nation to produce medical supplies to battle the coronavirus pandemic.
NCTO has been at the forefront of the efforts to deploy resources, converting production lines to manufacture urgently needed medical supplies like face masks and their textile components, to address the critical need for personal protective equipment and other medical and sanitation supplies in the fight against the coronavirus.
These unnecessary tariff concessions would benefit importers and retailers at the direct expense of manufacturers on the front lines of the COVID-19 response and send a demoralizing message.
Tariff deferrals would severely exacerbate ramifications for the U.S. economy, manufacturers and workers and open the floodgates for imports.
If the U.S. government makes tariff concessions during this crisis, it will be inviting a virtual tsunami of imports further devastating domestic manufacturing as it attempts to regain its footing.
We urge the administration to abandon any moves to defer tariffs on finished products. It would only serve to allow importers to exploit the current crisis, while dealing a severe blow to U.S. manufacturing and its workers.
Source: ncto.org
How the coronavirus travels through the air
How the coronavirus travels through the air has become one of the most divisive debates in this pandemic.
As the coronavirus pandemic continues, many people are now overthinking things they never used to think about at all. Can you go outside? What if you’re walking downwind of another person? What if you’re stuck waiting at a crosswalk and someone is there? What if you’re going for a run, and another runner is heading toward you, and the sidewalk is narrow? Suddenly, daily mundanities seem to demand strategy.
Read the full story: https://www.theatlantic.com/health/archive/2020/04/coronavirus-pandemic-airborne-go-outside-masks/609235/
Source: https://www.theatlantic.com/
Follow the Atlantic’s COVID-19 coverage: https://www.theatlantic.com/category/what-you-need-know-coronavirus/
Blue Box touts HVAC disinfection protocol for minimizing spread of coronavirus
Blue Box Air, LLC, is touting its disinfection protocol for combating the potential threat for the Coronavirus within commercial HVAC systems. The company’s disinfection protocol was developed to address the expected impact that many healthcare and other commercial buildings will be experiencing once as coronavirus.
Blue Box’s patented process for how to clean and disinfect the heat transfer coils within the commercial HVAC system is designed to optimize building energy efficiency and improving indoor air quality. By changing the formulation and developing a new disinfection protocol, Blue Box will focus on regular treatment of the HVAC systems heat transfer coils, which will involve a combination of using enzymes and chlorine dioxide to disinfect the coils.
Blue Box’s new disinfection protocol involves a process of removing existing biofilm deposits using its enzyme treatment formulation, then injecting Chlorine Dioxide through the coils to ensure that no microbial growths are able to survive. Blue Box has begun piloting its new disinfection protocol in New York City and is beginning to roll out its new service throughout the United States, including Los Angeles, San Francisco, Dallas, Miami, Chicago, Atlanta, and Washington DC.
Source: blueboxair.com
Oerlikon to supply 2-beam meltblown system to Asian manufacturer; opens its testing lab for production of face mask materials
A leading Asian large-scale manufacturer of manmade fibers and polymers has invested in a new Oerlikon Nonwoven meltblown system. The recently signed contract includes a 2-beam system for manufacturing filtration nonwovens – predominantly for medical products such as face masks – with a nominal capacity of up to 1,200 tons of nonwovens a year. The commercial production launch has been scheduled for the fourth quarter of 2020.
The 2-beam system has an operating width of 1.6 meters and is equipped with the new patented Oerlikon Nonwoven electro-charging unit. Electro-charging the filter nonwovens allows the manufacture of sophisticated EPA- and HEPA-class filter media as well as media that comply with the requirements of N95-, FFP2- and FFP3-class respiratory masks.
The demand for filtration nonwovens for medical applications has risen tremendously across the globe since the outbreak of the coronavirus epidemic, presenting all manufacturers with huge challenges. “We are currently receiving inquiries from all over the world for our system concepts,” said Ingo Mählmann, Ph.D., vice president of sales & marketing for Oerlikon Nonwoven. “To improve the supply situation, we have changed our prioritization in favor of considerably shorter delivery times for meltblown systems, so that customers can now be supplied even faster and also with very short lead times.”
A meltblown system will be commissioning at the site of a leading Western European nonwovens producers as early as the second quarter of 2020. This system will be deployed exclusively in the manufacture of nonwovens for respiratory masks.
Due to the current state of emergency with regards to the local supply of face masks, Oerlikon Nonwoven is currently using its own laboratory system to produce electrostatically charged filter media, which are being sent to local small businesses and companies for the manufacture of face masks. “We are thrilled to be making a contribution towards fighting the pandemic, particularly in the local vicinity of our production site in Neumünster”, adds Rainer Straub, head of Oerlikon Nonwoven.
Source: oerlikon.com/en/brands/nonwoven/
Related article:
When the unexpected happens, are today’s filtration systems ready for the challenge?
Berry expedites roll out of meltblown capacity in EMEIA market
Berry Global Group, Inc. has advanced its investment in an additional specialty meltblown asset to produce high-efficiency filtration media serving the EMEIA markets. Current projections are for commercial production to start in June 2020.
This investment is targeted to meet increased demand and customer growth and will be focused on premium applications, such as FFP2 (N95) and FFP3 (N99) for industrial face mask and cabin air filtration markets. The new line will be equipped with Berry’s proprietary charging technology to deliver optimal filtration efficiency and pressure drop at lower basis weights.
As the largest manufacturer of nonwoven fabrics and one of the world’s leading plastic packaging suppliers, Berry makes materials for face masks and protective healthcare apparel to packaging for food preservation and disinfecting products, many of which have seen a demand surge in the fight against COVID-19.
“As a market leader in that space, we had been planning to add more capacity shortly after our latest investment in Asia came on line. The opportunity to support the fight against COVID-19 accelerated our decision,” said Cedric Ballay executive vice president and general manager for Europe in Health, Hygiene, and Specialties for Berry. “Our ability to be agile will benefit our customers and our communities.”
Source: berryglobal.com
WPT Nonwovens acquires two lines, readies facility to begin production of surgical & N95 masks
WPT Nonwovens (Beaver Dam, KY) announced it will begin production of surgical masks and N-95 respirator masks, responding to requests from state and local officials looking for Kentucky manufacturers willing to assist with the production of supplies needed for the COVID 19 response.
According to Travis Robbins, WPT Nonwovens Vice President and General Manager, the company has moved forward with the purchase of two fully-automated machines, one for producing surgical masks and one for producing N-95 respirators. It has dedicated 5,000 square feet of its Beaver Dam facility to set up these two new production lines. However, the company said the raw materials needed to produce the masks has been challenging with price gouging on raw materials required to make the N-95 respirators.
Through the supply chain relationships developed by WPT Nonwovens, the company was able to source the necessary materials from a reliable and reputable supplier at an affordable cost. Robbins says that he is confident WPT Nonwovens will be able to supply needs at a price well below what the current market can offer, and that the company will be positioned to run 24/7 production to meet volume demands.
WPT Nonwovens expects to produce 100,000 surgical masks per day, and 35,000 N-95 mask per day. It expects the surgical masks will be ready to ship by May 1, and the N-95 masks will be ready by June 1.
Source: wptnonwovens.com
FEMA issues call for medical supplies, PPE to support COVID-19 response
- To sell medical supplies or equipment to the federal government, please submit a price quote under the COVID-19 PPE and Medical Supplies Request for Quotation. Full details can be found in the solicitation (Notice ID 70FA2020R00000011).
- This solicitation requires registration with the System for Award Management (SAM) in order to be considered for award, pursuant to applicable regulations and guidelines. Registration information can be found at www.sam.gov. Registration must be “ACTIVE” at the time of award.
- If you have medical supplies or equipment to donate, please provide us details on what you are offering.
- If you are a private company that wants to produce a product related to the COVID response – email nbeoc@max.gov.
- If you are interested in doing business with FEMA and supporting the response to COVID- 19 with your company’s non-medical goods and/or services, please submit your inquiry to the Department of Homeland Security (DHS) Procurement Action Innovative Response Team (PAIR) team at DHSIndustryLiaison@hq.dhs.gov.
Source: fema.gov
Considering alternatives to minimize the impact of the face mask shortage
An editorial published March 20 in JAMA requested creative ideas on how to reuse the N95 face masks, as well as how to make alternatives to commercial masks. The innovation on display convinced surgeon Ed Livingston, a coauthor of the editorial and an editor at JAMA, that “this is the biomedical engineering community’s Apollo 13 moment.”
In this fast-moving emergency, it’s unclear which homespun efforts will help the most. The following article considers how to best conserve the PPE that we have and how to make more.
Read the full story: https://www.sciencenews.org/article/coronavirus-covid-19-ppe-face-mask-shortages-creative-solutions
Source: sciencenews.org
How 3M Plans to Make More Than a Billion Masks By End of Year
Andrew Rehder, manager of 3M Co.’s respirator mask factory in Aberdeen, S.D., got the call from headquarters on Tuesday, Jan. 21. He gathered about 20 managers and supervisors into a conference room, where they sat, unworried, less than 6 feet apart. Rehder told them that a new virus was spreading rapidly in China and that 3M was expecting demand for protective gear to jump.
The Aberdeen plant had already ramped up production of respirator masks in response to demand from first responders battling wildfires in Australia and contending with a volcano in the Philippines. Now, Rehder told his charges, Aberdeen would shift to “surge capacity.” Idle machinery installed for precisely this purpose would be activated, and many of the plant’s 650 employees would immediately start working overtime. “We knew it wouldn’t be a two-week blip, it would be longer,” Rehder says. “But I had no idea.”
This is 3M’s moment, one for which the staid, 118-year-old Minnesota manufacturing giant—the maker of Post-its, Scotch tape, touchscreen displays, and scores of other products—has been preparing for almost two decades.
Read the full story: https://www.bloomberg.com/news/features/2020-03-25/3m-doubled-production-of-n95-face-masks-to-fight-coronavirus
Source: bloomberg.com
Honeywell increases production of N95 face masks to support COVID-19 response
Honeywell is ramping up production of N95 masks in the United States to support the COVID-19 response. The production boost will come from the Honeywell factory in Smithfield, Rhode Island, which also produces UVEX safety glasses.
The N95 face masks will be delivered to the U.S. Department of Health and Human Services for use to support health, safety and emergency response workers.
Honeywell expects the new mask production line in Smithfield will create at least 500 jobs. Recruiting, hiring and training manufacturing workers will begin immediately.
Source: honeywell.com
Klopman commits to produce 700k face masks per month
Klopman, a European manufacturer of protective fabrics, has committed to produce up to 700,000 protective masks per month, which are sterilizable and reusable up to 50 times, according to the company. Due to its capability in the production of fabrics for the medical and health sectors, the company has been included in Italy’s list of “essential” organizations.
The masks will be made from a double layer of fabric to ensure maximum protection and comfort while in contact with the nose and mouth. A first batch of 10,000 masks will be donated to the health authority of Frosinone and to the hospital “Fabrizio Spaziani,” with a rapid response supply chain in place to serve the needs of public and private operators.
In order to ensure a barrier effect with low particle release, Klopman said its masks will be based on the Vectron 8200 fabric, which is usually used in electronics, cleanrooms and in hospitals due to its characteristics, including a hydro/oil repellent finish to enable domestic and industrial washing at 75°C.
Source: klopman.com
Reifenhäuser Reicofil converts its own meltblown test lines to facemask production
The German machinery and plant manufacturer Reifenhäuser Reicofil has temporarily converted two of its test plants to full production in response to the coronavirus pandemic. The lines installed in the company’s nonwovens technology center, which are otherwise exclusively used for research and development as well as customer trials, have been producing meltblown material for the production of urgently needed facemasks.
Until further notice, the meltblown lines will be operated in 4-shift operation 24/7. The daily output is sufficient for up to one million facemasks. Reifenhäuser said trial operations will be almost completely suspended during this period.
The meltblown material from the nonwoven technology center is already sold out for the next five weeks. However, Reifenhäuser continues to look for opportunities to strengthen the local supply during this crisis. The company is in close contact with associations, authorities and other companies. Material for other medical protective clothing could also be produced at short notice, according to Reifenhäuser.
In related news, Reifenhäuser Reicofil is shortening the delivery time for meltblown lines to 3.5 months, with the aim of supporting the production of the crucial middle material layer for face masks. Supplies of face masks have been severely stressed in the face of the COVID-19 response.
Dr. Bernd Kunze, CEO of Reifenhäuser Reicofil, said, “In situations like the current one, we gladly depart from customary procedures. Sticking to standards in a non-standard situation is out of place. It goes without saying that we will do everything in our power to serve the needs quickly and in the accustomed high quality.”
The first contract with the new delivery time has already been concluded. The 1.6 meter wide Reicofil Meltblown line is scheduled to start operation in August 2020. With an annual output of 550 metric tons, the plant will produce H99 filter material for up to 1.8 million face masks a day.
Source: reifenhauser.com
Ford joins 3M, GE in speeding up ventilator, respirator production
Ford Motor Co said it was working with General Electric’s healthcare unit and 3M Co to speed up production of ventilators for patients and respirators for healthcare workers as the coronavirus pandemic escalates.
USTR requests input on lifting ‘Section 301’ duties on Chinese imports of medical-care products
In order to facilitate the U.S. response to COVID-19, the Office of the U.S. Trade Representative (USTR) has announced that it is now accepting comments from interested parties on the possibility of removing some of the additional “Section 301” duties that have been applied on various Chinese imported medical-care products.
According to USTR, each submission must specifically identify the particular product of concern and explain precisely how the product relates to the response to the COVID-19 outbreak. For example, the comment may address whether a product is directly used to treat COVID-19 or to limit the outbreak, and/or whether the product is used in the production of needed medical-care products. Comments may be submitted regarding any product covered by the action in the investigation, regardless of whether the product is subject to a pending or denied exclusion request. However, submissions are limited to comments on products subject to the tariff actions and relevant to the medical response to the coronavirus.
Source: inda.org
For more information, click here.
BYD opens face mask and bottled disinfectant plant
BYD created what it describes as the world’s largest mass-produced face masks plant, which is running at full capacity and is able to produce 5 million masks and 300,000 bottles of disinfectants per day. This allows the firm to help alleviate severe shortages that have affected hospitals and agencies across China in the face of the global COVID-19 outbreak.
On February 8, the newly-built production lines in one of BYD’s industrial parks in Shenzhen started to produce these critical supplies, with hundreds of staff working both day and night shifts along with machines working around the clock.
In late January, BYD began to assist in the production of masks and disinfection gels to tackle the growing COVID-19 outbreak. A special task force was appointed by BYD chairman and president Wang Chuanfu, consisting of leaders from different business divisions and more than 3,000 engineers involved in research and development, design, processing and other roles.
The task force moved in less than two weeks to finish work that normally takes two months, completing both the R&D and manufacturing process of mask production equipment within seven days, with the completion of R&D for medical-grade hand sanitizers in six days. Supplies were shipped to medical staff dealing with the COVID-19 response in China.
Source: byd.com
EU takes action to procure ventilators, masks and medical equipment
European Parliament is working with member states to ensure that the EU can buy ventilators, masks and other medical equipment to be put at the disposal of hospitals across the EU.
Last week, the Commission set up a scheme to gather medical equipment (through rescEU) so that the necessary supplies to combat COVID-19 can quickly get to member states facing shortages of equipment. This equipment is needed to treat infected patients, protect health care workers and help slow down the spread of the virus.
Parliament is working with member states to swiftly approve 40 out of 50 million EUR for intensive care medical equipment such as ventilators and personal protective equipment, such as reusable masks.
Member states are also joining forces under the Joint Procurement Agreement to buy personal protective equipment, respiratory ventilators and items necessary for coronavirus testing. Working together in this way will give them a stronger position on the world market.
Textile and apparel brands to build supply chain for emergency manufacturing of medical facemasks
American apparel brands and textile companies are responding to a White House request for medical supplies, building a supply chain to fast-track the manufacturing of medical face masks.
Parkdale Inc.– the largest yarn spinner in the U.S. headquartered in North Carolina—helped lead the effort to build the coalition with Hanesbrands, Fruit of the Loom and six other companies to set up a manufacturing supply chain and begin ramping up production of the masks.
The coalition consists of Hanesbrands and Fruit of the Loom, often competitors in the marketplace, who are banding together for the greater good of a nation facing one if its most monumental challenges.
American Giant, Los Angeles Apparel, AST Sportswear, Sanmar, America Knits, Beverly Knits and Riegel Linen are also part of the coalition working tirelessly to respond to a national emergency in the nation’s time of need.
Dr. Peter Navarro, assistant to the President and director of the White House Office of Trade and Manufacturing Policy, worked with the coalition and helped expedite the production of these masks. The first face masks have been approved by the U.S. Department of Health and Human Services.
The companies expect to begin production on Monday and will make the first deliveries by mid-week.
If companies are interested in dedicating resources to help the cause, please reach out to the National Council of Textile Organizations at kellis@ncto.org
NCTO is a Washington, DC-based trade association that represents domestic textile manufacturers, including artificial and synthetic filament and fiber producers.
Source: ncto.org
Related Story: “NC textile mill ‘heeds call of nation,’ gears up to make 10 million face masks per week”
U.S. Textile and Nonwoven Associations Urge Government to Deem Manufacturing Facilities “Essential”
U.S. textile and nonwoven associations issued a joint statement today urging federal, state and local governments to deem textile and nonwoven manufacturing facilities as “essential” when drafting “Shelter in Place” orders in response to the COVID-19 crisis. The statement was sponsored by the National Council of Textile Organizations (NCTO), INDA (Association of the Nonwoven Fabrics Industry) and Industrial Fabrics Association International (IFAI).
Our associations recognize the serious challenges our elected officials, health administrators and others are facing when issuing orders to protect communities across the country and we understand the necessity for leaders to enforce a ‘Shelter in Place” order or quarantine orders.
Our members make a broad range of inputs and finished products used in an array of personal protective equipment (PPE) and medical nonwoven/textile supplies, including surgical gowns, face masks, antibacterial wipes, lab coats, blood pressure cuffs, cotton swabs and hazmat suits. These items are vital to the government’s effort to ramp up emergency production of these critical supplies.
If workers who produce these goods are not granted an “essential” exemption from “Shelter in Place” and other quarantine orders to go to their manufacturing and distribution facilities, it will cause major disruptions in the availability of these goods. This will create significant hardship to healthcare providers and consumers across the country who depend on steady and stable supplies of these critical items.
We are asking the administration and state and local authorities to provide greater certainty and clarity for our companies and employees and ask for a clear exclusion of our manufacturing operations from “Shelter in Place” orders as the textile and nonwoven products that we make in the U.S. play an essential role in mitigating the shortages of critical supplies. Such a designation will help us avoid disruptions of vital goods and services during this challenging time.
Source: inda.org, ifai.com, ncto.org
COVID-19 Has Caused A Shortage Of Face Masks. But They’re Surprisingly Hard To Make
Both the masks made for medical personnel and for consumer purchase require a once-obscure material called melt-blown fabric. It’s an extremely fine mesh of synthetic polymer fibers that forms the critical inner filtration layer of a mask, allowing the wearer to breath while reducing the inflow of possible infectious particles.
“We’re talking about fibers where one filament has a diameter of less than one micron, so we are in the nano area,” said Markus Müller, the sales director at German company Reicofil, a major provider of melt-blown machine lines.
And there’s now a global shortage of melt-blown fabric due to the increased demand for masks — and the difficulty in producing this material.
Related article: https://www.filtnews.com/when-the-unexpected-happens-are-todays-filtration-sytems-ready-for-the-challenge/
Across Asia, countries race to boost face mask supplies
At a face mask factory just outside the South Korean capital of Seoul, workers are churning out 300,000 masks a day – and it’s still not enough.
3M taps regional suppliers to meet soaring demand for masks
The U.S. Department of Health and Human Services intends to buy 500 million N95 respirators over the next 18 months for the Strategic National Stockpile (SNS), the nation’s supply of pharmaceuticals and medical supplies.
Source: https://www.reuters.com/article/us-health-coronavirus-3m-idUSKBN20S214
Squabble over mask shortage erupts as coronavirus spreads
At a visit to 3M in Minnesota, Vice President Mike Pence noted the company can currently only produce 4 million FDA-blessed surgical masks per month.
Source: https://www.politico.com/news/2020/03/05/coronavirus-mask-shortage-122152
U.S. to Ramp Up Mask Production, But China Is Bottleneck for Raw Materials
Honeywell International Inc is the other major U.S. mask producer, which is aiming to ramp up production.
3M can’t confirm Pence comments about making more masks
Jennifer Ehrlich, a 3M communications manager said in an e-mail late Saturday that any information about government contracts for respirators would have to come from the Office of the Vice President. However, Ehrlich added, “Just to clarify, we are not yet under contract for the volume mentioned today. However, we are preparing to respond to the U.S. administration’s request for a proposal for respirators. 3M continues to maximize production at its manufacturing facilities in the U.S. and around the world for all types of N95 respirators.”
Source: http://www.startribune.com/3m-can-t-confirm-pence-comments-about-making-more-masks/568354132/
US mulls using sweeping powers to ramp up production of coronavirus protective gear
The Trump administration is considering invoking special powers to rapidly expand domestic manufacturing of protective masks and clothing to combat the coronavirus in the U.S., two officials told Reuters. The biggest producers of face masks in the United States include 3M Corp and Honeywell International Inc.
Health and Human Services (HHS) Secretary Alex Azar said at a congressional hearing on Wednesday that China controls “a lot of the raw materials as well as the manufacturing capacity” related to face masks. Very little of this stuff is apparently made in the (United) States, so if we’re down to domestic capability to produce, it could get tough,” the DHS official told Reuters.
3M ramps up N95 respirator production as demand surges from global coronavirus outbreak
From the streets of Beijing to Milan Fashion Week, people around the world are turning to protective masks and respirators to try and reduce the risk of infection amid the global outbreak of coronavirus.
Demand is so high that it’s increasingly difficult to order respirators on e-commerce platforms like Amazon.
“We’re seeing outbreaks develop in new countries every day. But even the countries where there isn’t a widespread outbreak are working really hard to prepare right now, in case they do have that situation,” Dr. Nikki McCullough, 3M’s global head of safety, told CNBC.





![Figure 1: Heat Exchanger Proventics GMBH.[22]](https://www.filtnews.com/wp-content/uploads/IFN_2_2024_crimpedmicrofiberyarns_Fig.-1-Heat-exchanger-225x125.jpg)







