In the biopharmaceutical industry, stainless steel equipment has always been a staple for processing various biologics. Procedures for cleaning and sterilizing stainless steel equipment are validated and have been considered a standard within the industry. Commercial scale manufacturing of biopharmaceuticals still continues to adopt stainless steel equipment over single-use (SU) technology, mainly due to economies of scale, range of batch size, and convenience. In the case of large scale production of recombinant protein, production costs using stainless steel equipment can be as low as ≤ $100/gram, whereas SU systems can only meet production costs from $300 – $500/gram.1 Although the utilization of stainless steel has been generally accepted within the industry, there are still several difficulties when using stainless steel equipment for bioprocessing.
Cleaning and sterilizing in place is required to remove any microorganisms that can grow naturally in media and process equipment. These steps can require a large energy demand, often with temperatures above 120 C for long periods of time. This becomes very time consuming, increasing overall batch cycle times and turnaround times between product campaigns. As a result, units are generally over-sized to overcome this. Stainless steel processing facilities generally consume a larger footprint and can take much longer to bring a new facility online.2
The emergence of SU technology has benefitted the industry by providing versatile, disposable solutions that can significantly reduce time and costs that are normally involved in the implementation of stainless steel bioprocessing. SU technology reduces the need for CIP/SIP. Plastic containment bags are pre-sterilized and validated before installation. Bags are generally pre-sterilized via gamma irradiation. Disposable devices lower the energy demand since high temperature steam and solvents are no longer required. Floor space requirements are also greatly reduced when implementing SU systems. Infrastructure requirements are no longer necessary, such as hard-piped plumbing and on-site water-for-injection plants, thus an SU system can be employed in a smaller setting, which decreases investment and construction costs while allowing for a simpler design that accelerates the process of bringing a new bioprocessing facility online.2
An industry shift
The cost to produce medications has been steadily increasing, which has pressured many manufacturers to trim production costs. Rising competition has also contributed to this increase with the growing development of biosimilars, which are less expensive alternatives that are almost identical to branded biologics.3 The deceleration of life-saving drug discovery and commercialization has also stiffened up competition over the years.4 It is becoming more difficult for health-care professionals to advise original brand biologics to insurance companies over biosimilars that are more affordable and provide indistinguishable benefits. As the industry grows, smaller developed markets are being approached that require smaller capacity demands.5 Contract development and manufacturing organizations are also growing to supplement production at the developmental stage. All of these factors have fueled the push towards SU technology. With smaller batch sizes, SU systems can be designed for smaller markets with little capital cost and greater versatility in a dynamic industry that seems to be constantly evolving. Over recent decades, the biopharmaceutical industry has continued to grow at an annual revenue growth rate of greater than 12 percent, with over an estimated 85 percent of pre-commercial scale production adopting SU systems.2
“It is becoming more difficult for health-care professionals to advise original brand biologics to insurance companies over biosimilars that are more affordable and provide indistinguishable benefits.”
SU requirements in cell harvesting
Upstream bioprocessing in cellular fermentation has been the quickest to adopt SU technology, with one of the major influencers being the increase of product titers. Titers have been steadily increasing and are currently averaging 3.4 g/L in both clinical and commercial scale operations.6 This is done by enriching media, improving cell productivity, increasing cellular mass, and lengthening cell duration. As a result, the percentage of solid impurities increase and must be filtered upstream.6
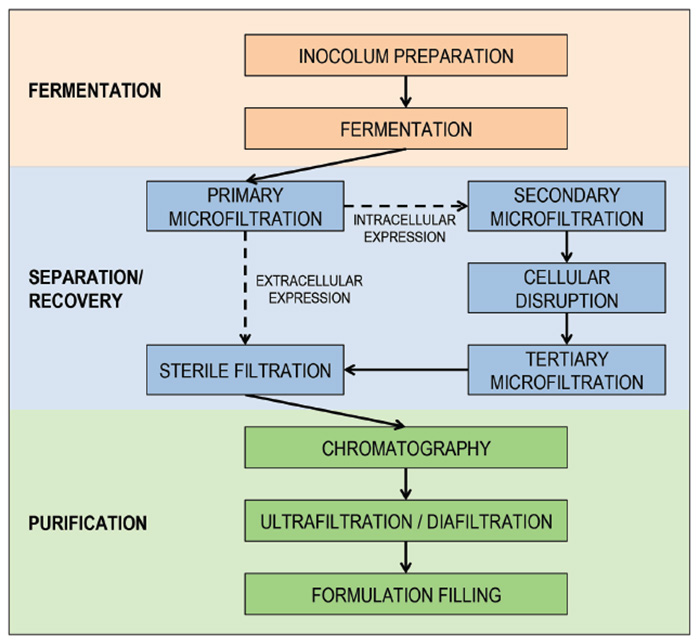
This has encouraged smaller bioprocessing demands that can achieve the same or even higher product output. These higher cell densities, which are achievable through mammalian and microbial cell lines can pose to be challenging for upstream filtration.7 SU filtration technologies used for harvesting cell bodies and debris must be able to handle high solids without breaking down the equipment in between batches. As a result, most equipment is purposely over-sized. They must operate under low shear to prevent rupturing the cells and releasing undesired proteins that can upset and hinder downstream processing. Mammalian cell cultures, which constitute ≥ 66-70 percent of the usage for biopharmaceutical-manufactured products, have no cellular walls and thus are very sensitive to shearing.2 The material of construction of the contact layers must also be compatible with the product. To ensure the product will not be contaminated, the SU manufacturer must perform leachable and extractable studies. Protein adsorption onto the plastic contact layer can also occur, which should be prevented when designing the filter. The full understanding of leachables and extractables is one of the leading reasons that companies are still hesitant to adopt SU technology.7
Multi-cycle SU filter
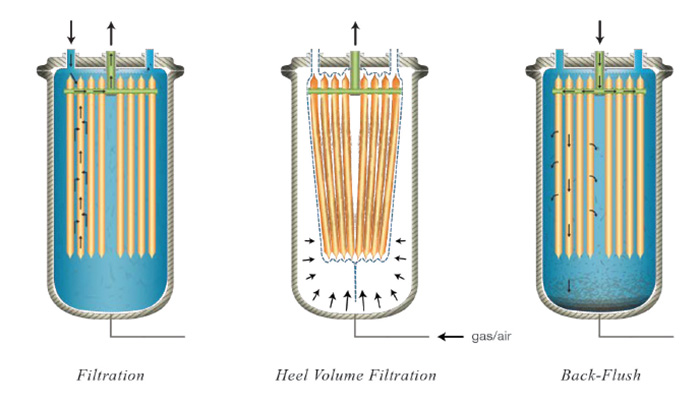
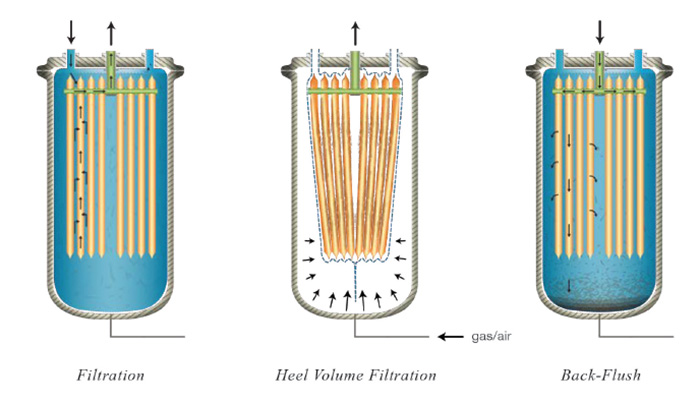
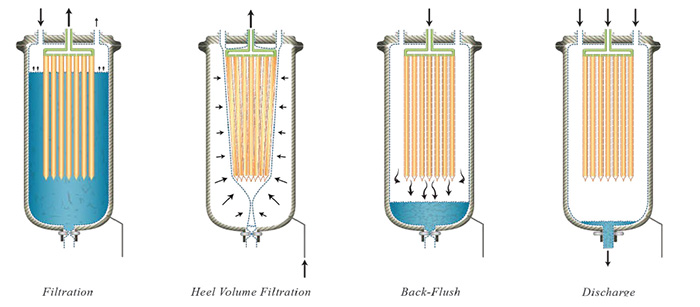
A novel filter solution for upstream microfiltration in biopharmaceutical processes has recently emerged. Common technologies employed in upstream cell harvesting include centrifugation, tangential-flow filtration, and depth filtration, often times coupled together in larger scale operations. This new concept in single-use filtration separates solids from suspension using dead-end filtration to deposit a cake. Flat filter elements are packed close together inside a multi-layered plastic bag enclosure to provide room for solid depositions up to 8 mm thick and to maximize surface area. The plastic bag is designed with multiple layers to be able to withstand chemical environments, undergo high pressures, and reduce gas permeation. The plastic filter bag is installed in a pressure vessel to allow the bag to be compressed via gas to dewater the cake. This enables filtration of the liquid heel so that there is virtually no hold-up left in the filter chamber. The filter elements are constructed in HDPE with an average pore size between 0.8 – 1 µm. These elements are flexible and conform to the shape of the bag when external pressure is applied. These elements are also back-washable via a back- flush tank which uses pressurized filtrate to discharge the deposited solids from the filter media and let them settle to the bottom of the filter bag. This enables the filter to perform multiple filtration cycles prior to disposal since solids can accumulate from cycle to cycle until the bag is full. Once full, the bag can be compressed to recover the hold-up volume, and the bag containing the solid waste can be removed and disposed of safely. In-situ filtration, cake washing, dewatering, and discharging provide a simple operation that reduces equipment costs and increases overall throughput.
This compact design significantly reduces disposal volume per weight of waste generated. For cell harvesting in extracellular protein expression, the volume of the containment under the filter elements can be increased to handle very high cell loads. Due to the shape and size of cells, filtration normally takes place with the addition of diatomaceous earth and/or cellulose to improve flow rates. Under this normal cake filtration, cells undergo relatively lower shear stress versus other filtration technologies, ensuring the integrity of the cells are maintained and that intracellular impurities are not released into the product.
In some processes, the filtered cells need to be discharged as thickened suspensions to pump downstream. In intracellular protein expression, the spent media must be replaced with a buffer solution to remove undesired impurities before undergoing cell lysis. An enhancement to the new SU design under consideration here incorporates an opening at the bottom for slurry discharge. This significantly extends the lifetime of the filter bag and increases filtration capacity. After cells are harvested and washed with a buffer solution, the filter elements can be back-flushed to transfer the concentrated cells downstream to be lysed. Disrupting the cells releases the desired proteins into the buffer solution and the lysed broth is re-filtered to remove the cell debris, isolating the lysate containing the desired product to send downstream to purification.
“This new concept in single-use filtration separates solids from suspension using dead-end filtration to deposit a cake. Flat filter elements are packed close together inside a multi-layered plastic bag enclosure.”
In continuous bioprocessing of cells, the cultures require continual supply of media to optimize growing conditions, as well as continuous removal of metabolites and product to prevent a toxic environment. This process allows for high-density cultures that maximize productivity in small bioreactors and increase titer levels. This SU design offers a solution to retain the cells in a recirculating loop off the bioreactor, while separating and replacing the supernatant. Perfusion bioreactors can sustain cell cultures for several weeks, which requires a robust, continuous filtration technology to be able to handle the high cell loads while maintaining continuous high product output.
Final thoughts
The growth of SU technology in the biopharmaceutical industry has pushed the innovation for new filtration devices that not only comply with the stringent regulations from several overseeing health organizations, but also provide a more efficient and safe operation for users. Costs for manufacturing new drugs are increasing with rising competition, so it is becoming more important for biopharmaceutical manufacturers to find solutions that are cost-effective and versatile. SU filtration upstream has been a major focus in the transition from stainless steel to disposable technologies. Fermentation processes are developing to increase cell densities, productivity, and cell loads, all of which increase the demand for upstream filtration.
SU filters must be designed to be pre-sterilized, to have full understanding of leachables and extractables per the application and must be highly durable to be able to handle high pressures. Design considerations should also be taken to reduce the hold-up volume and minimize overall disposable waste volume. The unique design of a multi-cycle SU filter offers a fully enclosed filter with high filtration area to handle high cell loads via dead-end cake filtration. With back-washable filter elements, solids can be discharged to settle at the bottom of the bag for multi-cycle use, thereby increasing the capacity of the filter, especially when dealing with solids that can have high resistance. These filters offer a new solution that can be used in various upstream biopharmaceutical processes to protect downstream equipment while reducing overall costs.
References
- Rader, R.A., “Biosimilars Paving The Way For Cost-Effective Bioprocessing,” Biosimilar Development, Aug. 23, 2017.
- Langer, E. S., and Ronald R.A., “Biopharmaceutical Manufacturing Is Shifting to Single- Use Systems. Are the Dinosaurs, the Large Stainless Steel Facilities, Becoming Extinct?” 23 Oct. 2018.
- Rathore, A., “Follow-on protein products: scientific issues, development and challenges,” Trends Biotechnol. Dec. 2009, pp. 698–705.
- Walsh, G., “Biopharmaceutical benchmarks 2010,” Nat. Biotechnol. 2010, pp. 917–924.
- Shukla, A. A., and U. Gottschalk. “Single-Use Disposable Technologies for Biopharmaceutical Manufacturing,” Trends in Biotechnology, 2012.
- Langer, E.S., et al, “15th Annual Report and Survey of Biopharmaceutical Manufacturing Capacity and Production,” BioPlan Associates, April 2018, 511 pages.
- Schmidt, S. R., et al. “Single-Use Depth Filters: Application in Clarifying Industrial Cell Cultures,” BioProcess International, Jan. 2017.