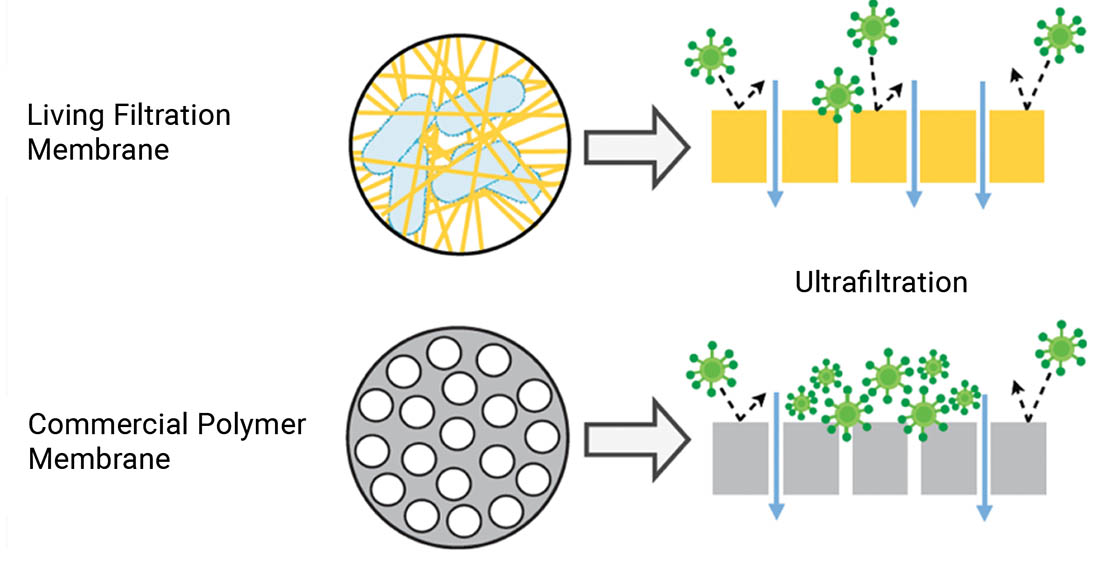
Composed of Bacterial Cellulose and Native Microorganisms, LFMs Demonstrate Antifouling Properties
As the need for safe water continues to expand across the world, membrane filters continue to be excellent prospects for increasing access to safe drinking water. However, the operation of membrane filters is limited by fouling (particularly, biofouling), and accessibility is limited by expensive and non-sustainable fabrication techniques.
Fouling plagues membranes by reducing efficiency and increasing maintenance and costs. Biofouling is particularly problematic due to the self-replicating nature of microorganisms and their secretion of extracellular polymeric substances, a sticky microbial matrix that make biofilms difficult to remove. Living filtration membranes (LFMs) are a sustainable membrane technology grown using symbiotic cultures of bacteria and yeast (SCOBYs), popularized by the fermented tea beverage, kombucha.
An LFM was compared to a commercial mixed cellulose esters (MCE) filter with a similar nominal pore size. Although the MCE membrane was more hydrophilic and had a smoother surface, surface properties that, in general, may improve fouling resistance for certain particles, more rapid flux decline and live biomass were observed with the MCE membrane for all three water sources tested.
We suggest that the LFM’s resistance to biofouling may be due to the proliferation of native bacteria, Acetobacter, which produces acetic acid, a known antibiofilm and antibacterial agent. Here are the highlights of the study, but the full article may be read for free online at https://pubs.acs.org/doi/10.1021/acsestwater.1c00169.
The Need for Safe Water
Globally, contaminated drinking water is linked to the deaths of nearly 2,000 children per day. Membrane filters are a promising technology for increasing the access to safe water because they are compact, versatile, and provide a physical barrier for many contaminants. The ultrafiltration category of membranes is of particular interest for rural settings and developing countries because they can effectively remove turbidity, bacteria, parasites, and some viruses while still operating at relatively low pressures.
The Hurdles of Membrane Filtration and Biofouling 101
Access to membrane filters is severely limited by chemical use during manufacturing and cost, while the operation of membrane systems is hindered by fouling, specifically biofouling. In filtration, biofouling describes the deterioration of performance due to the attachment and growth of microorganisms as a biofilm.
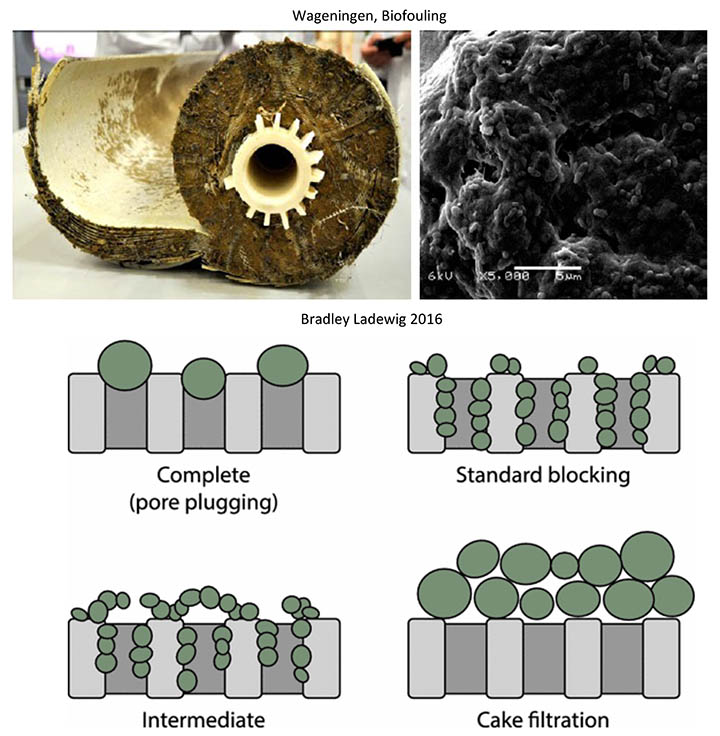
Ultrafiltration membranes primarily filter water by size exclusion: straining suspended particles larger than their pores. Inherently these pores eventually become “clogged” in several ways. This phenomenon is known as fouling, which is widely considered to be the most substantial issue in the design and operation of membrane filtration processes. As fouling occurs, hydraulic resistance is increased, making the process drastically less efficient. This can result in lower flux, decreased permeate quality, higher energy and chemical costs, and more frequent membrane replacement. Foulants can be biological (e.g., bacteria), colloidal (e.g., clays), organic (e.g., oils) or inorganic (e.g., minerals). Larger particles can plug pores and accumulate on the membrane surface, forming a cake layer – while smaller particles may adsorb and constrict the pore from within.


Though all types of fouling usually play a role, biofouling often becomes the dominant mechanism after long-term operation. Biofouling is also particularly unique from other types of fouling because the self-replicating nature of microorganisms makes the biofilms exceptionally persistent, even with the pretreatment of feedwater. These microorganisms also secrete extracellular polymeric substances (EPS), which provides most of the biofilm matrix’s cohesion, adhesion, and mechanical strength that makes them especially difficult to remove. EPS is responsible for many of the challenges involving biofouling. For example, EPS actually promotes the bulk of hydraulic resistance, not the microbes themselves.
Attachment of microorganisms is one of the first steps in biofilm formation. Membrane surface properties can influence attachment and the initial subsequent fouling. It has been demonstrated that smooth, hydrophilic, and net-neutral surfaces tend to best resist biofouling. A large portion of membrane research has focused on developing materials, techniques, and chemical treatments that allow for these antiadhesive and antifouling surface properties. While several of these technologies show promise, many of them are complicated and chemical-intensive, which makes them expensive, unsustainable, and inaccessible for the people that need them the most. Additionally, since most surfaces are eventually colonized, it has been suggested that more focus should be placed on materials which inhibit growth rather than adhesion.
There is a demand for simple solutions for biofouling control, which could include shifting the paradigm away from the killing of biofilms to the coexistence with biofilms.
Enter Living Filtration Membranes
Living Filtration Membranes (LFMs) were recently developed at Montana Technological University. They are composed of bacterial cellulose and native microorganisms from a Symbiotic Culture of Bacteria and Yeast (SCOBY). The SCOBY is produced in the brewing process of the popular fermented tea beverage, kombucha. LFMs can be grown within 10 days using only tea, water, sugar, vinegar, and a starter culture. All materials for producing LFM’s are not only inexpensive and widely available, but also biodegradable and safe for human consumption – a huge difference from how most other filtration membranes are fabricated.
As water filters, LFMs have demonstrated performance in the range of commercial ultrafiltration membranes, with a 90% rejection of 30nm particles. LFMs also displayed a multitude of unique properties, including the means to heal after being damaged. Many of these distinct features can be attributed to the living native microorganisms. The majority of prokaryotes comprising the LFM SCOBY are reported to be from the genera Acetobacter and Gluconobacter. These strains belong to the family Acetobacteriaceae, which are characterized by their unique ability to oxidize various sources of organic carbon and produce acetic acid.
Relevance of Acetic Acid
Acetic acid is present in relatively high concentrations within kombucha and has been used for thousands of years as an antimicrobial agent, dating back to the ancient Greek physician, Hippocrates. Acetic acid has not only been shown to restrict the growth of a broad array of both gram-negative and gram-positive bacteria, but has also exhibited the ability to completely eradicate foreign biofilms in some cases.
Method Synopsis
Raw water was collected from each of Butte, Montana’s three drinking water treatment plants: Basin Creek Reservoir, Moulton Reservoir, and The Big Hole River. The water was pretreated to imitate plant conditions at the time of filtration. Immediately following pretreatment, the water was sampled for a suite of analytes, including temperature, conductivity, and pH, as well as nutrients such total and dissolved carbon, nitrogen, nitrate + nitrite, and phosphorus.
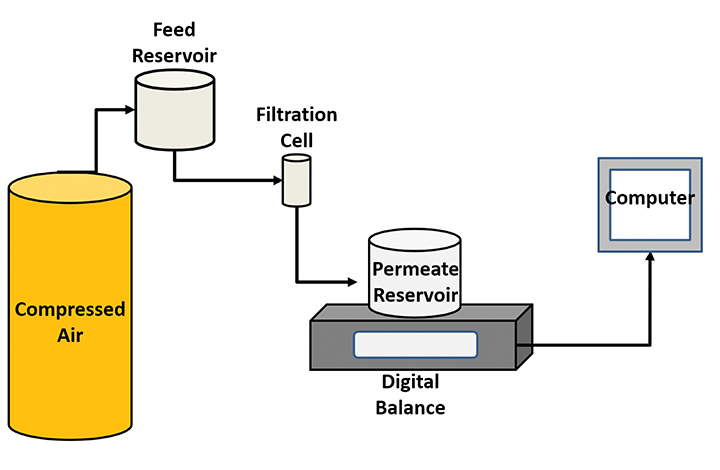
Living Filtration Membranes (LFMs) were produced using a method from prior publications (Eggensperger, 2019; Holland, 2020) and the bench scale ultrafiltration experiments were carried out using the same schematic. Mixed cellulose esters (MCE) membranes were purchased from Millipore Sigma. New membranes were used for each test. After each test, the initial stages of biofilm formation on the LFM and MCE membrane surfaces were quantified using confocal laser scanning microscopy and the software COMSTAT 2.1.
Three biofouling-critical physical surface properties were assessed for both the LFM and MCE: wettability/contact angle (a measure of surface hydrophilicity or hydrophobicity), surface topography (a measure of surface roughness), and zeta potential (a measure of surface charge). For the LFM only, DNA was also extracted, sequenced, and analyzed.
Key Findings and Discussion Surface Properties
Both membranes were found to be hydrophilic, but the LFM was significantly more hydrophobic than the MCE membrane. More hydrophilic ultrafiltration membranes are generally considered to have an increased resistance to fouling due to the hydrophobic nature of many foulants (thus, foulants may be repelled). Because the LFM was significantly more hydrophobic than the MCE membrane, it was surmised that the LFM could actually be more susceptible to fouling.
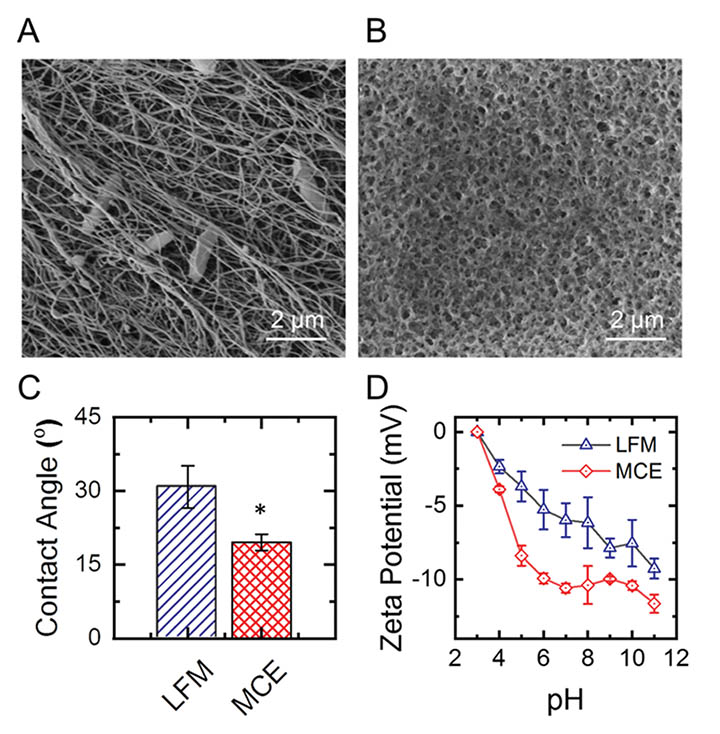
Surface topography of LFM and MCE membranes was analyzed qualitatively via SEM to determine if topography could play a role in fouling (Figure 1, A and B). These images indicate that LFMs have a substantially rougher and more heterogeneous surface, while MCE membranes appear much smoother. MCE membranes may also appear to have smaller pores. However, due to LFM nanofibers growing in dense, stacked sheets: the LFM experiences a more selective nominal pore size of 30 nm compared to the MCE membranes 50 nm. Rough membrane surfaces have demonstrated increased fouling compared to smooth surfaces, especially by colloids. Similar to the results of membrane hydrophilicity, surface topography results appear to indicate that the LFM could be more susceptible to fouling.
The MCE membrane exhibited a more electronegative surface charge than the LFM (Figure 1, D). However, both surfaces still had a relatively negative charge. Some surface water foulants like proteins, microbes, and natural organic matter are negatively charged in solution, and charge repulsion between the membrane and negatively charged particles may occur on the membrane surface. Due to the pHs of the feedwater, charge repulsion was unlikely in Big Hole and Moulton experiments but more likely in Basin Creek.
Based on the surface topography and hydrophilicity of the membranes, it was expected that the LFM would experience more exacerbated fouling than the MCE membrane.
Flux
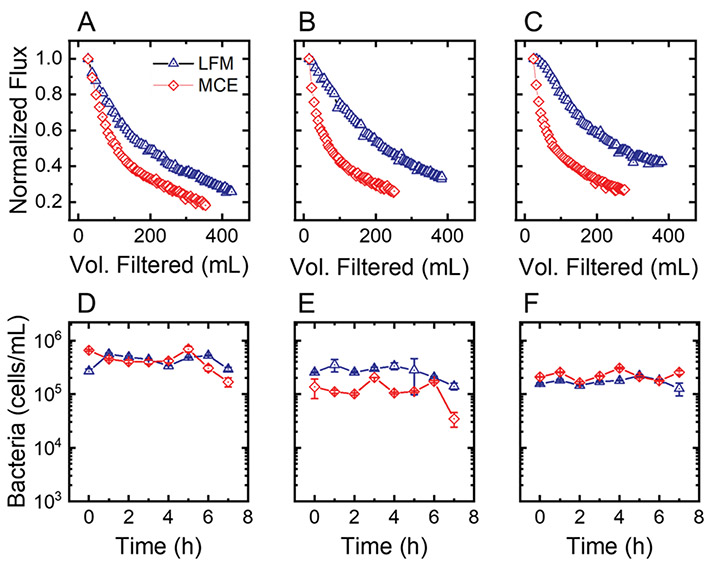
Flux is one of the most important operating parameters for membrane filters because it represents performance in terms of volume of water filtered per area of membrane. Despite the indication of fouling-unfavorable surface properties, overall, the LFM’s flux outperformed the commercial membranes for the three water sources tested by approximately 19%, 33%, and 40%. When the literature was reviewed, fouling for MCE membranes appeared consistent with that of the commercial polymer membranes used in other studies, whereas the LFMs appeared to have a resistance to fouling.
Microbial Communities and Feed Water Analytes
The LFM bacterial genus was 97% Acetobacter and 2.4% unclassified, with several other taxa <0.2%. Acetobacter are commonly reported as the dominant genus within kombucha, reaching especially high concentrations at a pH near 3.
Acetobacter are characterized by their ability to perform oxidative fermentation: a process in which a variety of organic carbon sources (sucrose, glucose, ethanol, etc.) are partially oxidized in the presence of oxygen into acetic acid and other metabolites. Acetic acid has a long history of use for its antimicrobial properties, and has more recently exhibited the ability to mitigate and eradicate biofilms. The predominance of Acetobacter suggests that the potential for acetic acid generation in the LFM may contribute to its anti-fouling effects.
Membrane filters are great prospects for combating the water crisis, but their application is limited by biofouling and their accessibility is restricted by expensive and chemical-intensive fabrication.
Biofilm Analysis
When the post-filtration biofilms were analyzed using a confocal microscope, it was shown that LFMs had less live biomass than MCE membranes for all three water sources. These differences were statistically significant in Big Hole (p < 0.001) and Moulton (p = 0.042), but not in Basin Creek (p = 0.074). This finding is consistent with the flux data, which showed that, while the LFM outperformed MCE in all waters, the performance difference was most profound when filtering water from Big Hole and Moulton. The Moulton water had a significant difference in live biomass between membranes (p = 0.042); however, it is important to note that biofouling is not the only type of fouling that can occur: in ultrafiltration with natural waters, organic fouling can contribute significantly to fouling.
In this study, dead biomass appeared to be less influential on membrane performance than live biomass, which may be due to dead biomass not being able to reproduce or generate new EPS, the material that mainly increases hydraulic resistance. In Moulton water, where the membrane flux difference was most drastic, there was no statistical difference in dead biomass between the two membranes (p = 0.958).
Putting It All Together
Thousands of people per day die globally from drinking contaminated drinking water. Membrane filters are great prospects for combating the water crisis, but their application is limited by biofouling and their accessibility is restricted by expensive and chemical-intensive fabrication. LFMs can overcome some of these challenges by being inexpensive and sustainable to produce while still treating water effectively. Despite several fouling-unfavorable membrane-surface properties, the LFM’s flux outperformed the commercial membranes for the three water sources tested by approximately 19%, 33%, and 40%. LFMs experienced less live biomass on membrane surfaces, indicating a resistance to biofouling. We suggest that the large population of native Acetobacter within the LFM may discourage the growth of biofouling bacteria: Acetobacter oxidize organic carbon and produce acetic acid, an effective antibiofilm and antimicrobial agent. In waters with higher concentrations of TOC, the LFM’s experienced larger performance differences and significantly less live biomass than the MCE membranes.
Further, a publication using preliminary LFM and MCE data suggested that this resistance to fouling may reduce lifecycle environmental impacts of ultrafiltration considerably compared to commercial polymer membranes (Jiang et al., 2020). In addition to filtration membranes, bacterial cellulose is also being investigated as a wound dressing, where fibers grown from Acetobacter specifically might be useful for mitigating biofilms and infections within the field of medicine.
- Flux, one common performance metric for membrane filters, is the volumetric flow rate of water per sur-face area of membrane (e.g., liters per square meter).
- (p < .001) based on a 95% confidence interval.
- pHs of the feedwaters were near 3.0. Please read the entire article for more information on the feedwater characteristics.