The societal and, in some cases, legal requirement of wearing facemasks in public to protect the user from the SARS-CoV-2 coronavirus has led to an unprecedented increase in demand for high-quality masks. With demand on the rise, there is also an increased need to ensure what is being sold to the public meets the relevant standards for filter media particle penetration.
There have been numerous examples of masks being sold in the U.S. and Europe that do not meet the standards that they claim. The purpose of this article is to discuss the importance of accurately testing masks for efficiency and the best techniques to perform the tests.
Size and shape matter
One important factor to be aware of is not all masks are of the same size and shape. However, all masks are required to meet the requirements of relevant standards, regardless of size and shape. This means the testing process must be flexible enough to support the range of shapes and sizes of different mask types.
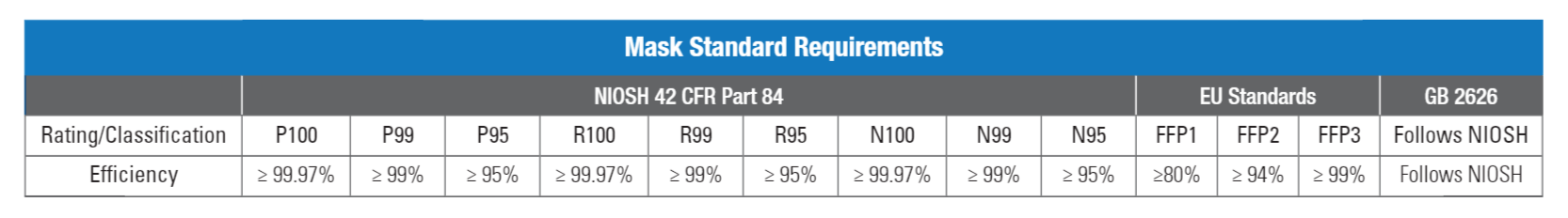
Masks sold in the U.S., Europe and China must meet the standard requirements unique to the region in which they are sold (NIOSH 42 CFR Part 84 (U.S.), EN13274-7:2019 (Europe), and GB2626 (China). And while the standards are similar, they do have some subtle differences that must be addressed.
An expanding mask landscape
At the same time there is work being done to develop “community” mask standards for the general public and workers not employed in high-risk and/or frontline medical scenarios. ASTM International just released a new specification for barrier face coverings (ASTM F3502-21), which balances particle efficiency with breathability of the material.
Typically, the higher the efficiency the lower the breathability (or, in other words, how easy it is to breathe with the mask on). ASTM F3502-21 masks do not have the same performance as an N95 mask, but are considerably better than cloth masks, which do not offer sufficient protection and, worse, can provide a false sense of security.
The maximum efficiency requirement for the ASTM F3502-21 mask is 50%, with less than 5mm H20 for breathability. The intent is to save the N95-quality masks for front-line workers who need them the most and separately offer a suitable low-cost, comfortable and effective mask option for the general population.

Test prep – putty vs. beeswax
Proper prepping of the mask for efficiency testing is extremely critical to ensure accurate results. This point cannot be overstated, as poorly prepping a mask can lead to false results.
Likewise, the quality of the fixture used to test the mask is critical to ensure accuracy. The mask adapter must be 100% free of any leaks in the structure to ensure accurate results.
Air Techniques International of Owings Mills, Maryland, has developed a unique method that easily allows for the testing of various types of masks. This method incorporates the use of putty instead of beeswax. Both putty and beeswax perform the same task, providing an airtight seal for the mask to the substrate. This is critical, as an accurate test requires all the aerosol being generated to pass through the mask and not underneath or around the edges. If this happens, they will get artificially large penetration values.
- The putty can be used with a variety of mask types such as cup and duckbill styles that are common with N95, KN95 and FFP masks. It can be used for mask made to the most common standards such as NIOSH, EN13274, GB262 and ASTM F3502.
- Putty, once applied can be easily removed or adjusted to ensure a complete seal. If the putty is carefully removed after testing it is possible for it to be re-used on the next mask to be tested.
Putty is advantageous for prepping facemasks for a variety of reason, including:
- Easy to handle
- Easy to apply
- Easy to clean
- No fumes and non-toxic
- Readily available (Amazon, etc.)

The main drawback of putty is that it is slightly more expensive than the traditional method of using beeswax. Also, there has been a preference by some for beeswax because beeswax is recommended by the U.S. National Institute for Occupational Safety and Health in its recommended practices for filter testing procedures.
Common drawbacks of beeswax for prepping facemasks include:
- Beeswax needs to be heated, so there is a risk of burns.
- An electrical outlet is required to heat the wax.
- Beeswax is not easy to handle when it is wet.
- Beeswax is difficult to clean, generally a pretty messy process.
- Very difficult for duckbill masks or masks that don’t lay completely flat
False tests
If expected results are not attained when testing a mask, it is important to check that the substrate and mask is fully sealed with putty. For proper testing results, all of the aerosols should be coming through the mask for a proper test. If there is a leak between the mask and substrate, then a false high-penetration result is likely. If this happens, check that the mask itself doesn’t have any obvious holes. Finally check the mask adapter for leaks.
Parting thoughts
Properly testing masks that are intended for consumers as protection against SARS CoV-2 (or other viruses) is critical for manufacturers to ensure their product does indeed meet the desired specification. All internationally recognized standards require testing of masks before being sold, so testing is a critical step in the overall manufacturing and QA process.