Research Project Aeropore Provides Innovative Test Methods
Many biological hazards such as viruses, bacteria, fungi and allergens being present as airborne infectious or allergenic particles had a low profile in aerosol research until the COVID-19 pandemic. At the beginning of the pandemic, the main control strategies focused on the disinfection of surfaces and hand hygiene rather than virus transmission via air, which turned out to play the most important role regarding the source of hazardous infections. Measures, such as ventilation or air purification and face masks, that lead to a reduction in the viral load in the air we breathe, are therefore considered as the most effective protective strategy against infectious airborne pathogens (e.g., Ebola, influenza, endemic coronaviruses or molds) and against airborne allergens such as pollen spread via air.
Status Quo: Air Filter Evaluation with Non-biological Aerosols
The greatly increased transparency on the topic of bioaerosols in the scientific community and social media caused a significant increase in research activities as well as the need for solution concepts on efficient reduction options of these airborne pathogenic aerosols in indoor air, and vice versa. Thus, many innovative concepts are developed to enable low-noise and energy-efficient reduction of pathogens in indoor air. However, the efficiency of air purification to remove airborne pathogens in a real indoor environment can still only be estimated to a very limited extent at present. While established test standards with precisely defined test germs and conditions have been developed for decades in the field of surface hygiene, there is currently a strong need in the field of air hygiene for test methods that can reliably measure and quantify the efficiency of measures to reduce infectious pathogens such as viruses in indoor air.
Testing with real biologically active viruses, bacteria or allergenic particles, especially at large scales can be challenging and complex. However, they could support better understanding of infection control and pathogen transmission, especially for risk minimization instruments such as filtration systems in health care settings.
In current routine standard operations, non-biological aerosols such as salt particles, oil-like droplets or fine dust are applied for air filter evaluation (ISO 16890, DIN EN 71460-1, ÖNORM/EN 149). Regarding the investigation of air cleaners, there are promising studies aiming at more realistic indoor test scenario using e.g. NaCl aerosols (Küpper et al., 2019). While these particles represent the size of viruses, nanometer-scale particle deposition is also largely related to the physicochemical properties of, for example viruses, which cannot be easily simulated by a saline aerosol. It becomes particularly difficult when it is not a matter of pure filtration, but when the viruses are to be inactivated (e.g., by UV light ionization or electrochemical processes). Here, realistic experiments with infectious pathogens cannot be substituted, since the determination of the infectivity of the particles plays an essential role.
Testing with Real Airborne Microorganisms

Testing with real biologically active viruses, bacteria or allergenic particles, especially at large scales can be challenging and complex. However, they could support better understanding of infection control and pathogen transmission, especially for risk minimization instruments such as filtration systems in health care settings. As working with very hazardous and harmful pathogens is often not possible, the application of less harmful microbial model strains is recommended in several aerosol studies.
One possibility to mimic pathogenic viruses are bacteriophages, which are bacterial viruses infecting specific bacterial host cells, which means, they are non-pathogenic to humans. Although these bacterial viruses reflect the properties of critical pathogens such as Ebola, influenza or various endemic or pandemic coronavirus strains quite well, they cannot infect humans, but just bacteria.
Thus, a high level of operational safety can be ensured. Bacteriophages might support and facilitate aerosolization studies and subsequently, prediction and estimation of behavior and reactivity of highly pathogenic airborne viruses.
As biological particles such as viruses behave differently and comprise detectable infectious potential compared to inert non-biological particles, it is important for the design and testing of filtration and ventilation systems to investigate the interactions between bioaerosols and filters as well as air conditioning and cleaning devices. These non-biological particles may represent the size of e.g. viruses but do not necessarily reflect the separation mechanisms on nanometric scale, which are often connected to physio-chemical characteristics of viruses, which cannot be simulated by an e.g. salt particle.
In general, it is known, that non-enveloped viruses are more persistent towards environmental stress such as temperature, relative humidity, irradiation via UV or disinfection agents, whereas in the case of enveloped viruses, additionally possessing a lipid membrane carrying, e.g. characteristic membrane spike proteins, are more sensitive towards environmental influencing factors. Furthermore, bacteria or fungal spores may change size and shape depending on cell membrane and wall characteristics as reaction environmental factors such as desiccation, temperature, humidity or the presence of surrounding carrier fluids, droplets or particles. The potential of applied bioaerosols lies furthermore in serving as extension tool to commonly used non-biological aerosols such as salt or DEHS particles for evaluation of potential reduction of biological risk by e.g. air filters or Heating, Ventilation and Air Conditioning (HVAC) systems.
What is Bioaerosol?
If an aerosol mixture contains biological material or microorganisms, the term bioaerosol or biological aerosol is used. Such a biological aerosol consists of living and non-living components or fragments such as pollen, fungi, bacteria and viruses. These differ in size:
- Size of fungal spores: most commonly observed size range of spores is 2 to 10 μm but can vary from around 1 to 50 μm
- Size of bacteria: 0.5 up to 4 μm is assumed depending on bacteria species as well as sampling and analysis techniques
- Size of viruses: 15-500 nm
- Size of pollen: average between 25 and 40 µm, subpollen particles (“Ubisch bodies” ~0.5 µm)
The Research Project AEROPORE
In the FFG-funded research project “Aeropore,” the Austrian Research Institute for Chemistry and Technology (OFI) together with the Center for Electron Microscopy Graz (ZFE), both members of ACR (Austrian Cooperative Research), have developed a novel filter test rig. This enables filter systems to be assessed not only on the basis of their permeability to particles in terms of number and size, but also according to their biological risk. Starting in autumn 2023, OFI expert Bernadette Führer will be researching in the project “AeroMobil” how this novel test facility can also be used in mobile applications.
Breathe Easy: Insight into the Research Project Aeropore
Within the research project “Aeropore” conducted at OFI, an independent testing and research institute in Austria and member of ACR (Austrian Cooperative Research), the subject was intensified. In the course of the project, it was possible to develop a pilot-scale simulation filter test rig (at right) for testing air filters with airborne biological hazards belonging to biological safety level 1 (BSL1).
Even before COVID-19 pandemic the topic air hygiene and biological safety played a major role for OFI. Thus, the development of reproducible and comparable methods allowing the performance of single-pass tests using bioaerosols (determination of filter passage, fresh “air mode”) for evaluation of air filters from a biological and immunological perspective could be enabled.
Whereas everything started with a focus on allergens, pollen and fungal spores, the testing scope has been extended further during the course of the project. Working with bioaerosols containing bacteria and viruses used for filter testing was enabled at OFI within a second step, whereas the COVID-19 pandemic definitely enhanced transparency and attention on the topic.
It is known, that handling airborne particles functioning as test bioaerosol can be challenging. Each microbial strain or bioparticle requires proper preparation, sampling, aerosolization and detection techniques due to different characteristics and structural features of the biological particles, especially in airborne state. In addition, capability to survive as well as to maintain specific biological activity in airborne state is required by applied microbes for being able to detect them.

As there are very strict safety regulations when working with highly pathogenic and infectious microorganisms such as SARS-CoV-2, influenza viruses, Ebola or Mycobacterium tuberculosis at laboratory, many research studies recommend safe and non-pathogenic models for human viruses.
To give an example, commonly used virus surrogates for aerosol experiments are MS2 bacteriophage and phi6 bacteriophage, which are also used at OFI for filter and air cleaner evaluation. MS2 bacteriophage, a well-known bacterial virus with an average size of 27 nm, was chosen as representative for a robust non-enveloped virus comparable to noroviruses. On the other hand, phi6 bacteriophage with an average size of 86 nm was selected as enveloped virus posing as a surrogate for Ebola virus or Coronavirus.
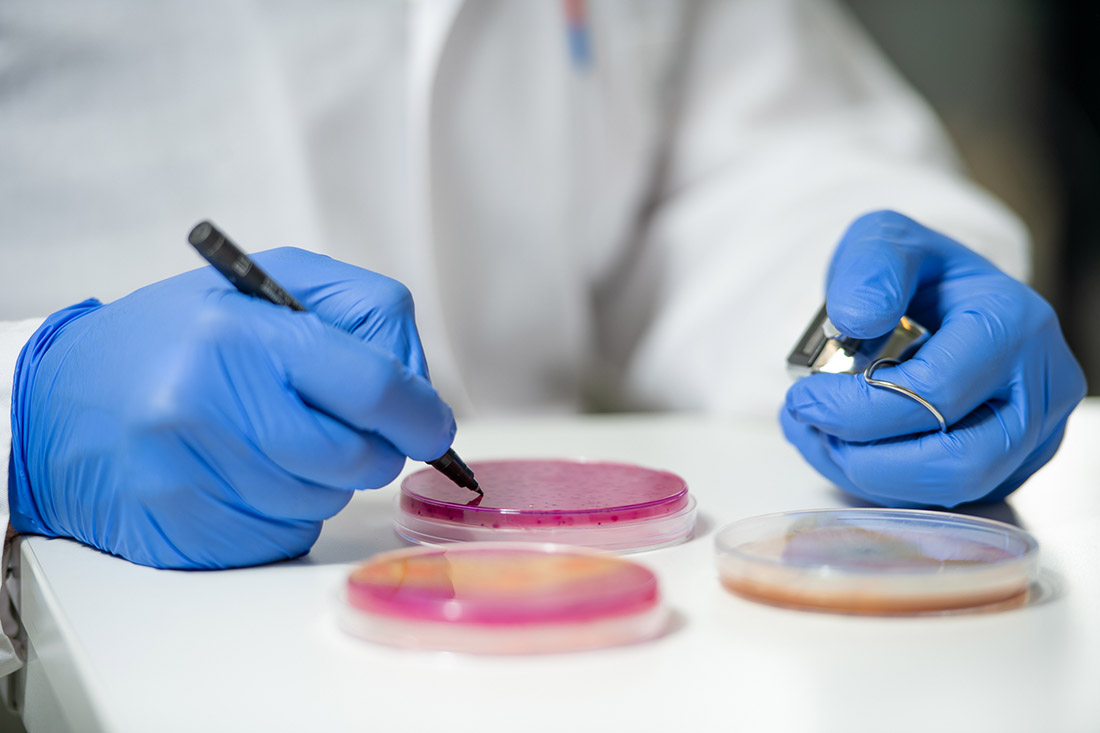
During method development, both viral surrogates were compared regarding their stability and recovery as airborne particles for testing air filters within OFI filter test rig. For checking the suitability of the selected strains as a stable bioaerosol recovery and viability after aerosolization into the test rig were investigated. Liquid aerosol generator from Topas GmbH was used for aerosolization of selected bioaerosols at different volume flow rates ranging from 40-300 m³/h for defined period of time per run. The nebulized airborne viruses sprayed into the test duct were sampled via collection filter media mounted at the end of the test duct followed by liquid extraction, which is just one of several bioaerosol sampling techniques.
The quantification of the viruses is usually done via double layer method by optically counting plaque forming units (PFU) being the golden standard for detection of bacteriophages. The method requires incubation of the host bacteria together with the sample solution containing the target virus at specific conditions. So-called plaques are formed by present bacteriophages as a result of infecting host bacteria, grown on agar plates containing nutrient media. For evaluation of retention capacity or filtration efficiency of air filters regarding hazardous bioparticles, tests with and without a test filter, (a cabin air filter) are done.
Following general issues were important to consider during development of test bioaerosols applied within a filter test rig:
- Stability and recovery of viruses, bacteria, fungal spores or allergenic pollen on surfaces.
- Stability and recovery of airborne particles in airborne state after aerosolization within whole test duct.
- Checking suitability of bioaerosol for single-pass tests for air filters, filter media and air cleaners.
More Research is Needed
The application of bioaerosols for a standardized air filter evaluation is still a black box within aerosol research and filter testing, which could be further opened for supporting estimation and improvement of infection control and prevention but also proper selection of a filter providing energy-saving performance within an effective filtration or air cleaning system. The implementation of biological methods within more realistic scenarios using selected infectious bioaerosols could be realized further within real-life test setups as, for example, within a car for evaluation of a car HVAC system.
In the future, OFI will deal with this topic even more intensively: in the autumn of 2023, the research project “AeroMobil” will start. The aim is to facilitate the assessment of air purification measures in different room concepts by developing a mobile test station.
References:
- Whitworth, C., Mu, Y., Houston, H., Martinez-Smith, M., Noble-Wang, J., Coulliette-Salmond, A., & Rose, L. (2020). Persistence of bacteriophage Phi 6 on porous and nonporous surfaces and the potential for its use as an Ebola virus or coronavirus surrogate. Applied and Environmental Microbiology, 86(17), e01482-20.
- Verreault, D., Marcoux-Voiselle, M., Turgeon, N., Moineau, S., & Duchaine, C. (2015). Resistance of aerosolized bacterial viruses to relative humidity and temperature. Applied and environmental microbiology, 81(20), 7305-7311.
- Küpper, M., Asbach, C., Schneiderwind, U., Finger, H., Spiegelhoff, D. and Schumacher, S. (2019). Testing of an Indoor Air Cleaner for Particulate Pollutants under Realistic Conditions in an Office Room. Aerosol Air Qual. Res. 19: 1655-1665. https://doi.org/10.4209/aaqr.2019.01.0029
- Miaskiewicz-Peska, E., & Lebkowska, M. (2012). Comparison of aerosol and bioaerosol collection on air filters. Aerobiologia, 28(2), 185–193. https://doi.org/10.1007/s10453-011-9223-1
- Després, V. R., Alex Huffman, J., Burrows, S. M., Hoose, C., Safatov, A. S., Buryak, G., Fröhlich-Nowoisky, J., Elbert, W., Andreae, M. O., Pöschl, U., & Jaenicke, R. (2012). Primary biological aerosol particles in the atmosphere: A review. In Tellus, Series B: Chemical and Physical Meteorology (Vol. 64, Issue 1). https://doi.org/10.3402/tellusb.v64i0.15598
- Souza, A. S. R., Fernandes, A. S., Vanderlei, L. C. D. M., Costa, A. A. R. D., Ferreira, A. L. C. G., Dubeux, L. S., … & Cabral Filho, J. E. (2021). Transparency in research and publications on COVID-19. Revista Brasileira de Saúde Materno Infantil, 21, 5-7.
- Tang, S., Mao, Y., Jones, R. M., Tan, Q., Ji, J. S., Li, N., … & Shi, X. (2020). Aerosol transmission of SARS-CoV-2? Evidence, prevention and control. Environment international, 144, 106039.
- Nazarenko, Y. (2020). Air filtration and SARS-CoV-2. Epidemiology and health, 42.



![Figure 1: Heat Exchanger Proventics GMBH.[22]](https://www.filtnews.com/wp-content/uploads/IFN_2_2024_crimpedmicrofiberyarns_Fig.-1-Heat-exchanger-225x125.jpg)






